Institute Announcements
Grant Will Fund M. Kerry O'Banion's Work on Space Travel & the Immune System
Tuesday, December 1, 2020
M. Kerry O'Banion, M.D., Ph.D. has been awarded $1.8 million from NASA to explore the effect space travel has on the immune system and bone marrow, and how that impacts brain function.
The grant is one of 21 research proposals recently awarded by NASA to help answer questions about astronaut health and performance during future long-duration missions, including crewed missions to the Moon and Mars.
Using simulated space radiation produced by particle accelerators at the NASA Space Radiation Laboratory at Brookhaven National Laboratory on Long Island, O'Banion and his team will examine tissue and cellular changes in genes, blood flow, and immune cell function in mice. Behavioral tests and computer-assisted imaging will also be used to quantify damage and inflammation in the brain.
O'Banion -- Professor of Neuroscience and Neurology in the Del Monte Institute for Neuroscience -- and colleagues previously worked with NASA on a study that showed exposure to a particular form of space radiation called high-mass, high-charged particles caused biological and cognitive changes in mice suggesting an accelerated risk for the development of Alzheimer's disease.
This time around, O'Banion will be working with Laura Calvi, M.D., an endocrinologist and co-director of the UR Multidisciplinary Neuroendocrinology Clinic. Her preliminary data found space radiation changes in bone marrow suggestive of a skewed phenotype, in which white blood cells are changed into a more inflammatory phenotype. Similar changes are found with aging. "This helps to bind a common hypothesis about dysfunction and degeneration in multiple systems, with the bone marrow communicating to the brain through the vasculature," O'Banion said.
Memories Create ‘Fingerprints’ That Reveal How the Brain is Organized
Friday, November 20, 2020
While the broad architecture and organization of the human brain is universal, new research shows how the differences between how people reimagine common scenarios can be observed in brain activity and quantified. These unique neurological signatures could ultimately be used to understand, study, and even improve treatment of disorders such as Alzheimer's disease.
"When people imagine similar types of events, each person does it differently because they have different experiences," said Feng (Vankee) Lin, Ph.D., R.N. "Our research demonstrates that we can decode the complex information in the human brain related to everyday life and identify neural 'fingerprints' that are unique to each individual's remembered experience." Lin is an associate professor in the University of Rochester Del Monte Institute for Neuroscience and School of Nursing and co-author of the study which appears in the journal Nature Communications.
In the study, researchers asked 26 participants to recall common scenarios, such as driving, attending a wedding, or eating out at a restaurant. The scenarios were broad enough so that each participant would reimagine them differently. For example, when researchers asked volunteers to vividly remember and describe an occasion involving dancing, one person might recall watching their daughter participating in a dance recital, while another may imagine themselves dancing at a Bar Mitzvah.
The participants' verbal descriptions were mapped to a computational linguistic model that approximates the meaning of the words and creates numerical representations of the context of the description. They were also asked to rate aspects of the remembered experience, such as how strongly it was associated with sound, color, movement, and different emotions.
The study volunteers were then placed in a functional MRI (fMRI) and asked to reimagine the experience while researchers measured which areas of the brain were activated. Using the fMRI data and the subject's verbal descriptions and ratings, researchers were able to isolate brain activity patterns associated with that individual's experiences. For instance, if the participant imagined driving through a red light in the scenario, areas of the brain associated with recalling motion and color would be activated. Using this data, the researchers built a functional model of each participant's brain, essentially creating a unique signature of their neurological activity.
The researchers were able to identify several areas of the brain that served as hubs for processing information across brain networks that contribute to recalling information about people, objects, places, emotions, and sensations. The team was also able to observe how activation patterns within these networks differed on an individual level depending upon the details of each person's recollections and imagination.
"One of the goals of cognitive science is to understand how memories are represented and manipulated by the human brain," said Andrew Anderson, Ph.D., with the Del Monte Institute for Neuroscience and co-author of the study. "This study shows that fMRI can measure brain activity with sufficient signal to identify meaningful interpersonal differences in the neural representation of complex imagined events that reflect each individual's unique experience."
In addition to expanding our understanding of how the brain is networked, the authors point out that many of the key regions they identified tend to decline in function as we age and are vulnerable to the degeneration that occurs in disease like Alzheimer's. The findings could lead to new ways to diagnose and study disorders associated with irregular memory deficits, including dementia, schizophrenia, and depression, and perhaps even personalize treatments and predict which therapies will be more effective.
Additional co-authors include Kelsey McDermott, Brian Rooks, Kathi Heffner, and David Dodell-Feder with the University of Rochester. The study was funded with support from the National Center for Advancing Translational Sciences of the National Institutes of Health and the URMC Clinical the Translational Science Institute.
Research to Treat Neurodegenerative Diseases Advances: URMC Start-up Acquired
Wednesday, November 18, 2020
Oscine Therapeutics -- a biotechnology company that is developing cell-based therapies for neurological disorders based on discoveries made at the University of Rochester Medical Center (URMC) -- has been acquired by Sana Biotechnology for undisclosed terms.
The research behind Oscine is based on decades of work in the lab of Steve Goldman, M.D., Ph.D., professor of Neurology and Neuroscience and co-director of the URMC Center for Translational Neuromedicine. Goldman's research has focused on understanding the basic biology and molecular function of the glial support cells in the central nervous system, devising new techniques to precisely manipulate and sort these cells, and studying how cell replacement could impact the course of neurological diseases. Goldman, who was Oscine's president and scientific founder, joins Sana as senior vice-president and head of Central Nervous System Therapy. He will also remain on the URMC faculty.
Sana Biotechnology, which has operations in Washington, Massachusetts, and California, was created in 2018 with a focus on developing and delivering engineered cells as medicines for patients. The company is led by a team of biotechnology industry veterans and supported by more than $700 million in investment. Last year, the company invested in Oscine's R&D in neurological disorders, in what remains the University of Rochester's largest-ever commercial spin-off.
The exclusive licenses for the portfolio of technologies and equity stake that the University of Rochester held with Oscine have been acquired by Sana. The University and Goldman may continue to receive significant licensing, milestone, and royalty payments from Sana going forward.
"The University of Rochester has been working closely with Dr. Goldman's lab and the Oscine team from its inception," said Scott Catlin, director of UR Ventures, the University's technology transfer office. "We are thrilled with the company's impressive progress and its acquisition by Sana and look forward to continue supporting the commercialization of Dr. Goldman's technologies."
Goldman's research focuses on support cells in the brain called glia. In many neurological diseases -- such as multiple sclerosis, Huntington's, and neuropsychiatric disorders -- these cells either disappear or malfunction. This ultimately leads to the motor, cognitive, and behavioral symptoms of these disorders. Goldman's lab has shown that replacing these sick cells with healthy ones can slow and even reverse disease progression in animal models of these diseases.
The Center for Translational Neuromedicine maintains labs in Rochester and at the University of Copenhagen in Denmark. Goldman's research for cell-based therapies has received relevant support from the National Institute of Neurological Disorders and Stroke, the National Institute of Mental Health, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Lundbeck Foundation, the Novo Nordisk Foundation, CHDI, and NYSTEM.
Imaging the secret lives of immune cells in the eye
Friday, October 9, 2020
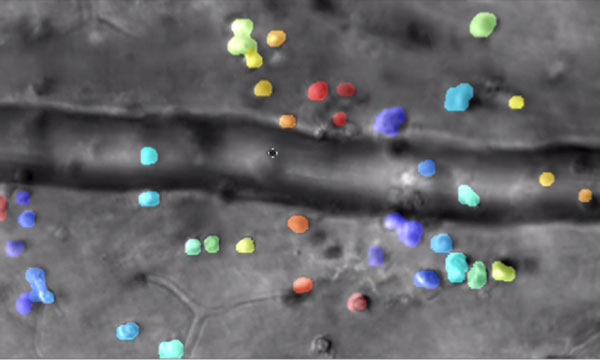
Captured by the lab of Jesse Schallek, assistant professor of ophthalmology and neuroscience, the image shows microscopic immune cells escaping a nearby blood vessel in response to inflammation. The color overlay shows computer detection of single cells that are tracked over time. (Courtesy of Schallek lab)
Rochester researchers demonstrate way to track the interactions of microscopic immune cells in a living eye without dyes or damage, a first for imaging science.
Combining infrared videography and artificial intelligence, the new technique could be a 'game-changer' for some clinical diagnoses as well as for fields like pharmaceuticals.
University of Rochester vision scientist Jesse Schallek can barely contain his excitement as he shares time-lapse videos showing immune cells moving through living retinal tissue at the back of an eye.
In one clip, immune cells crawl so slowly along the inside edge of a blood vessel that the video must be sped up 25 times to show their progress. Another cell slowly treads against the flow of blood in a vessel, like a salmon fighting its way upstream. Other immune cells leave the blood vessels and inch through the surrounding tissue, then congregate in a swarm, forming a beehive of activity.
Schallek and his vision lab at the University of Rochester Center for Visual Science and Flaum Eye Institute, have created a new microscopy technique, described in the journal eLIFE, that builds upon groundbreaking adaptive optics developed at the University more than 20 years ago.
Combined with time lapse videography and artificial intelligence software, the new technique enables researchers for the first time to noninvasively image and track—without labeling—the interactions of translucent immune cells within live retinal tissue in animals. Until now, the immune cells had to be labeled with fluorescent agents and often reinjected in order to image them—raising questions about how this might change the behavior of the cells. Another common, but limiting approach is to remove cells and study them with a microscope in a dish.
Nedergaard Honored for Alzheimer’s Research
Tuesday, September 15, 2020
Maiken Nedergaard, M.D., D.M.Sc. has been awarded the International Prize for Translational Neuroscience of the Gertrud Reemtsma Foundation for her research in the glymphatic system, the brain's unique waste removal process.
Nedergaard's research was recognized by the Foundation for findings that "offer new approaches for treatments and preventive measures for Alzheimer's and other neurodegenerative diseases."
First discovered by researchers in the URMC Center for Translational Neuromedicine in 2012, the glymphatic system piggybacks on blood vessels and pumps cerebrospinal fluid, washing waste from the brain. The accumulation of toxic proteins like beta amyloid are linked neurological disorders, including Alzheimer's disease. Nedergaard's lab has since gone on to show how sleep disruption, age, and injury can impair the brain's ability to effectively remove waste.
Nedergaard was presented the award at a ceremony at the Max Plank Society in Cologne, Germany on September 10.
2020 Convocation Award Winners
Tuesday, September 15, 2020
Let's all send congratulations to our graduate students and faculty for once again being recognized at Convocation.
Graduate Alumni Fellowship Award - Paige Nicklas
Irving L. Spar Fellowship Award - Maleelo Shamambo
J. Newell Stannard Scholarship Award - Michael Giannetto
Merritt and Marjorie Cleveland Fellowship - Victoria Popov
Outstanding Graduate Program Director - Anna Majewska, Ph.D.
Outstanding Graduate Course Director - Robert Stanley Freeman, Ph.D.
Rochester leads novel research project on how the brain interprets motion
Thursday, September 3, 2020
Major NIH award to study how the brain infers structure from sensory signals may have applications for disorders like schizophrenia and offer insights for artificial intelligence
Imagine you're sitting on a train. You look out the window and see another train on an adjacent track that appears to be moving. But, has your train stopped while the other train is moving, or are you moving while the other train is stopped?
The same sensory experience—viewing a train—can yield two very different perceptions, leading you to feel either a sensation of yourself in motion or a sensation of being stationary while an object moves around you.
Human brains are constantly faced with such ambiguous sensory inputs. In order to resolve the ambiguity and correctly perceive the world, our brains employ a process known as causal inference.
Causal inference is a key to learning, reasoning, and decision making, but researchers currently know little about the neurons involved in the process.
In order to bridge the gap, a team of researchers at the University of Rochester, including Greg DeAngelis, the George Eastman Professor of Brain and Cognitive Sciences, and Ralf Haefner, an assistant professor of brain and cognitive sciences, received a $12.2 million grant award from the National Institutes of Health for a project to better understand how the brain uses causal inference to distinguish self-motion from object motion.
The five-year award is part of the NIH's Brain Research through Advancing Innovative Neurotechnologies (BRAIN) initiative. The insights generated by the award, which also involves researchers at New York University, Harvard Medical School, Rice University, and the University of Washington, may have important applications in developing treatments and therapies for neural disorders such as autism and schizophrenia, as well as inspire advances in artificial intelligence.
"This NIH BRAIN Initiative Award is the biggest research award in the history of the Department Brain and Cognitive Sciences," says Duje Tadin, professor and chair of the department at Rochester. "It aims to solve the key question of how our brains interpret the information collected by our senses. This research builds on a longstanding strength of BCS of using computational methods to understand both behavior and underlying neural mechanisms."
Unraveling a complicated circuit of neurons
Causal inference involves a complicated circuit of neurons and other sensory mechanisms that are not widely understood, DeAngelis says, because "sensory perception works so well most of the time, so we take for granted how difficult of a computational problem it is."
In actuality, sensory signals are noisy and incomplete. Additionally, there are many possible events that could happen in the world that would produce similar patterns of sensory input.
Consider a spot of light that moves across the retina of the eye. The same visual input could be the result of a variety of situations: it could be caused by an object that moves in the world while the viewer remains stationary, such as a person standing still at a window and observing a moving ambulance with a flashing light; it could be caused by a moving observer viewing a stationary object, such as a runner noticing a lamppost from a distance; or it could be caused by many different combinations of object motion, self-motion, and depth.
The brain has a difficult problem to solve: it must infer what most likely caused the specific pattern of sensory signals that it received. It can then draw conclusions about the situation and plan appropriate actions in response.
Using data science, lab experiments, computer models, and cognitive theory, DeAngelis, Haefner, and their colleagues will pinpoint single neurons and groups of neurons that are involved in the process. Their goal is to identify how the brain generates a consistent view of reality through interactions between the parts of the brain that process sensory stimuli and the parts of the brain that make decisions and plan actions.
Developing therapies and artificial intelligence
Recognizing how the brain uses causal inference to separate self-motion from object motion may help in designing artificial intelligence and autopilot devices.
"Understanding how the brain infers self-motion and object motion might provide inspiration for improving existing algorithms for autopilot devices on planes and self-driving cars," Haefner says.
For example, a plane's circuitry must take into account the plane's self-motion in the air while also avoiding other moving planes appearing around it.
The research may additionally have important applications in developing treatments and therapies for neural disorders such as autism and schizophrenia, conditions in which casual inference is thought to be impaired.
"While the project is basic science focused on understanding the fundamental mechanisms of causal inference, this knowledge should eventually be applicable to the treatment of these disorders," DeAngelis says.
Krishnan Padmanabhan has publication in Frontiers in Computational Neuroscience
Monday, July 13, 2020
Top-Down Control of Inhibitory Granule Cells in the Main Olfactory Bulb Reshapes Neural Dynamics Giving Rise to a Diversity of Computations
Growing evidence shows that top-down projections from excitatory neurons in piriform cortex selectively synapse onto local inhibitory granule cells in the main olfactory bulb, effectively gating their own inputs by controlling inhibition. An open question in olfaction is the role this feedback plays in shaping the dynamics of local circuits, and the resultant computational benefits it provides. Using rate models of neuronal firing in a network consisting of excitatory mitral and tufted cells, inhibitory granule cells and top-down piriform cortical neurons, we found that changes in the weight of feedback to inhibitory neurons generated diverse network dynamics and complex transitions between these dynamics. Changes in the weight of top-down feedback supported a number of computations, including both pattern separation and oscillatory synchrony. Additionally, the network could generate gamma oscillations though a mechanism we termed Top-down control of Inhibitory Neuron Gamma (TING). Collectively, these functions arose from a codimension-2 bifurcation in the dynamical system. Our results highlight a key role for this top-down feedback, gating inhibition to facilitate often diametrically different computations.
NGP Student Honored with Edward Peck Curtis Award for Excellence in Teaching
Friday, May 22, 2020
Neuroscience graduate student Monique Mendes, M.S., has received the Edward Peck Curtis Award for Excellence in Teaching by a Graduate Student.
"I'm extremely proud of my students and what they have accomplished in and outside of the lab. I am incredibly fortunate to have been presented with opportunities to teach students throughout my Ph.D. I want to thank them because I have learned so much in the process," Mendes said.
Mendes was one of 13 graduate students to be honored with this award, which requires graduate students to have significant interaction with undergraduate students in the classroom or lab, and excel in advancing the teaching mission of the University by providing highly-skilled and innovative instruction.
"I was thoroughly convinced by the nomination submitted by the faculty that Monique is an outstanding educator with a bright future," Vice Provost and University Dean of Graduate Education Melissa Sturge-Apple, Ph.D., said. In presenting the award to Mendes virtually earlier this month, Sturge-Apple presented Mendes remarked "I'm grateful for all of your hard work and your mentoring and teaching which is central to the mission of our University, so I was so honored to give you this award. I wish I could do it in person."
During the presentation, Sturge-Apple read some of the nomination letters considered in the process:
"She [Monique] has a very didactic nature to her that is beautiful complimented by her enthusiasm and her vigor. She sets the setting naturally and her persistent work ethic is taught without words but through actions."
"As a younger black woman who wants to go into science and medicine I don't have very many people in my life who go into my field of interest and definitely not many who look like me, so Monique is a role model in that sense as well. She takes away some of the feelings of otherness that I had in certain situations and serves as a reminder that I can do this and I do belong."
"She has a passion that's contagious and she is clear and succinct in conveying information. She wants those around her to understand the material and to love it the same way that she does."
Mendes is a 5th year student in the Neuroscience Graduate Program and is studying the dynamics and kinetics of microglia self-renewal in the adult brain.
Two 2020 NGP Graduates Honored for Thesis Work
Friday, May 22, 2020
Rianne Stowell, Ph.D. was awarded the Wallace O. Fenn Award for her thesis that characterizes the dynamics of microglia, and the mechanisms regulating the function of these cells in different areas of the brain. This award is given annually to a graduating student who has performed especially meritorious research. According to her advisor Ania Majewska, Ph.D., the research that contributed to Stowell's thesis was published in a series of three manuscripts and two reviews. Stowell's work put microglia in the spotlight, as heterogeneous complex cells that are exquisitely tuned to activity in the brain. One of the main ¬- ¬and surprising - findings was that their activities are largely carried out in the quiescent or sleeping brain. This discovery has broad implications for understanding how microglia fit into the functions of the brain's networks and the development of novel therapeutics for neurological diseases where microglial function is likely altered. "The work highlights Stowell's strong independent streak and a great work ethic," Majewska said. "That, coupled with her innate intellectual abilities and creativity, results in a winning combination that will take her far in the future. This thesis is a great beginning to an incredibly promising scientific journey."
Dawling Dionisio-Santos, M.D., Ph.D. was awarded The Vincent du Vigneaud Award for his thesis work that was judged as superior and unique with the potential to stimulate and extend research in the field. According to Dionisio-Santos' advisor M. Kerry O'Banion, M.D., Ph.D., Dionisio-Santos moved his research in a more translational direction and initiated a series of experiments using glatiramer acetate, a drug currently prescribed for the treatment of multiple sclerosis. He discovered that, in addition to reducing amyloid plaque levels, glatiramer acetate also reduces tau pathology and improves behavioral performance, demonstrating clear translational relevance for patients with Alzheimer's disease. "Dionisio-Santos is a talented future physician-scientist," O'Banion said. "With outstanding potential based on his demonstrated ability to carry out complex experiments and analyses, develop new ideas and experiments based on thorough evaluation of the literature, and inspire others with his passion for wanting to better understand neurodegenerative diseases."
Maiken Nedergaard honored by American Stroke Association for dedication to stroke research
Monday, February 24, 2020
Maiken Nedergaard, M.D., D.M.Sc., co-director of the Center for Translational Neuromedicine, professor in the Departments of Neurology, Neuroscience and Neurosurgery, received the Thomas Willis Lecture Award from the American Stroke Association. The award honors Nedergaard's career of significant contributions to the basic science of stroke research.
The Nedergaard lab is dedicated to deciphering the role of neuroglia, cell types that constitute half of the entire cell population of the brain and spinal cord.
Last month, the lab published research showing that during a stroke the glymphatic system goes awry, triggers edema and drowns brain cells. In 2012, Nedergaard and her colleagues first described the glymphatic system, a network that piggybacks on the brain's blood circulation system and is comprised of layers of plumbing, with the inner blood vessel encased by a 'tube' that transports cerebrospinal fluid (CSF). The system pumps CSF through brain tissue, primarily while we sleep, washing away toxic proteins and other waste.
The Thomas Willis Award honors the prominent British physician credited with providing the first detailed description of the brain stem, the cerebellum and the ventricles, with extensive hypothesis about the functions of these brain parts. The award recognizes contributions to the investigation and management of stroke basic science.
Nedergaard was one of eleven leading scientists honored for their work by the American Stroke Association. The awards were given during the American Stroke Association's International Stroke Conference in Los Angeles.
Del Monte Institute for Neuroscience offers 2020 pilot funds
Tuesday, February 4, 2020
The Ernest J. Del Monte Institute for Neuroscience (Del Monte) is pleased to announce the availability of up to 21 pilot project awards (maximum budget of $50,000 per award) to support novel basic, clinical and translational projects in the neurosciences. These awards will be supported under 5 programs for 2020 and are open to all faculty members across both the Medical School and the Undergraduate Campus. Funds available for this year's program are $810,000.
The Schmitt Program in Integrative Neuroscience (SPIN) supports pilot and feasibility awards (up to $50,000 per award) for basic science and translational projects that advance our understanding of both normal and abnormal brain functioning (4-5 awards available).
The Harry T. Mangurian Jr. Foundation (MF) offers pilot and feasibility awards (up to $50,000 per award) for basic, clinical and translational projects that specifically support research on Autism Spectrum Disorder (ASD) (2 awards available).
The Rochester Center for Alzheimer's Disease Research (RCADR) supports pilot and feasibility awards (up to $50,000 per award) for basic science and translational projects that advance our understanding of Alzheimer's disease and related dementias (4 awards available).
Center for Health + Technology Clinical Neuroscience Pilot Program (CHET) offers pilot and feasibility awards (up to $50,000 per award) for clinical research projects that advance our understanding of areas of unmet need in clinical neuroscience (4 awards available).
University of Rochester Center for Advanced Brain Imaging and Neurophysiology (UR CABIN) offers pilot and feasibility funds (up to $10,000 per award) to support innovative, investigator-initiated basic and clinical neuroscience research using the PRISMA 3T magnet (up to 6 awards available).
For more information and to download the RFA, click here. Application submissions are due on Friday, May 1st.
Suzanne Haber Honored by Society of Biological Psychiatry for Research on Mental Disorders
Thursday, January 30, 2020
Suzanne N. Haber, Ph.D., Dean's Professor in the Department of Pharmacology and Physiology, will receive the Society of Biological Psychiatry's 2020 Gold Medal Award at the Society's 75th Annual Scientific Convention & Meeting in the spring. The award honors members of the Society whose significant and sustained work has advanced and extended knowledge on the neurobiology of mental illness.
Haber's lab investigates the cortico-cortical and cortico-basal ganglia systems in the brain. Her work demonstrates the specific hard-wired connections that are associated with normal decision making, emotional and cognitive control, and the connectional abnormalities in those circuits that are linked to a wide range of mental health disorders, including obsessive-compulsive disorder (OCD), drug abuse and addiction, schizophrenia, and motor control disorders such as Parkinson's disease. This work has played a key role in targeting and interpreting the effects of noninvasive and invasive therapeutic approaches for OCD and depression.
For the past ten years, Haber has led the Silvio O. Conte Center for Basic and Translational Mental Health Research at the University of Rochester. Funded by the National Institute of Mental Health, the Center uses translational approaches to probe the neurocircuitry that underlies neuromodulation for OCD, pinpointing specific abnormalities within the brain circuits that are associated with the disease. This information is being used to guide new treatment options for the three million-plus Americans who live with the disorder.
"Suzanne's seminal contributions to elucidating specific neural networks that control learning, decision-making, reward and motivation, and how pathologies associated with these neural communication hubs underlie multiple neurological, movement, and mental health disorders make her uniquely qualified to receive this prestigious career award," said Robert T. Dirksen, Ph.D., Lewis Pratt Ross Professor and Chair of the Department of Pharmacology and Physiology. "Her work is making a difference in the lives of individuals and families suffering from neurological and mental health disorders. We are extremely proud that she represents the University of Rochester as a Society of Biological Psychiatry Gold Medal Award winner."
The Society of Biological Psychiatry was founded in 1945 to emphasize the medical and scientific study and treatment of mental disorders. It's the oldest neuropsychiatry research society in America, currently made up of more than 1,500 members from across the United States, Canada, Europe and Asia. Members conduct research in areas spanning from basic cellular studies to clinical trials and prevention research.
Haber, who is also a professor of Neuroscience, Brain and Cognitive Science, and Psychiatry, will split the 2020 Gold Medal Award with Carol Tamminga, M.D. of UT Southwestern Medical Center.