News
McGrath Lab graduate student’s accidental exhale leads to improved DNA detector
Thursday, December 7, 2017
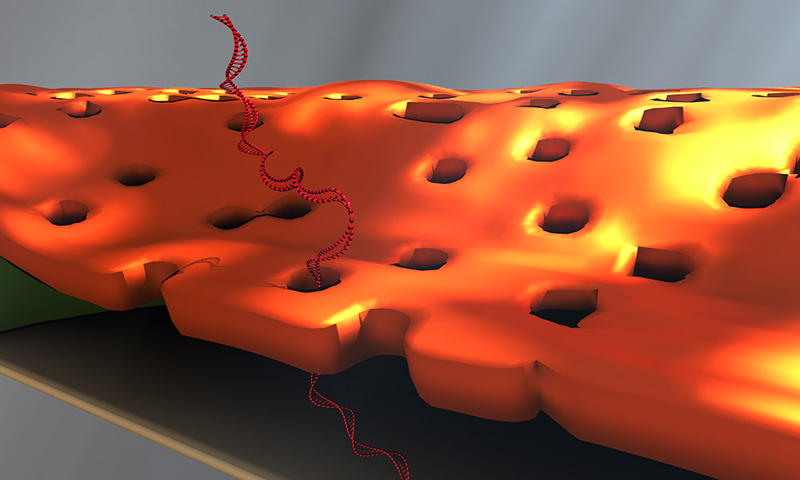
Doctoral student Greg Madejski's illustration of the layers comprising his new DNA detection device. (University of Rochester illustration / Greg Madejski)
Greg Madejski held his breath as he looked into the microscope, trying to weld two fingernail-sized chips together: a tiny chip containing a nanofilter on top of another chip with a DNA sensor.
It was frustrating work. The chips weren't making good contact with each other. Madejski gently poked at the chips, then peered over the top of the microscope.
And exhaled.
The sudden waft of warm air swept over the nanofilter, transferring it to the sensor—right on target. The "accident" led Madejski to an important insight: the water vapor in his breath had condensed on the device, causing the nanofilter to adhere ever so neatly to the sensor.
"It was like a really high-tech temporary tattoo that I created by accident; lick and stick!" says the PhD student in the lab of James McGrath, a professor of biomedical engineering at the University of Rochester.
And that's how water vapor became integral to the development and design of a novel device for detecting DNA biomarkers affiliated with disease. Created by McGrath's lab in collaboration with Professor Vincent Tabard-Cossa and graduate student Kyle Briggs at the University of Ottawa, the device is described in an article published online at Nano Letters. The article, and an image from Madejski's homemade animation of the device in operation, will be highlighted on the cover of the February 2018 print issue.
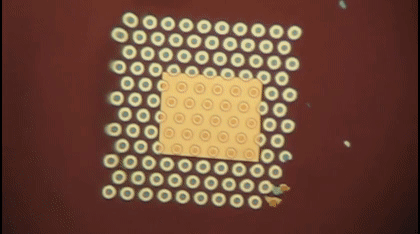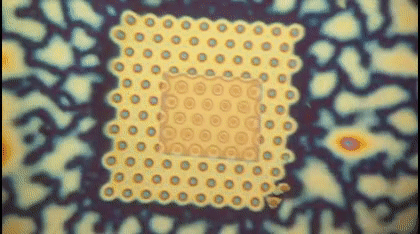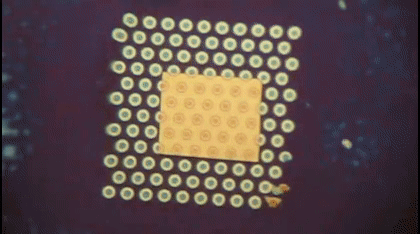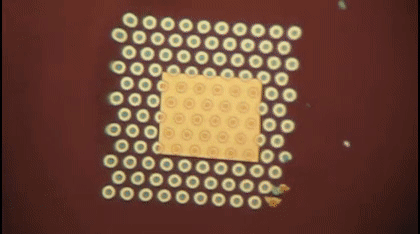
This animation shows, as graduate student Greg Madejski explains, the "thin films of water, seen as rainbow colors, swelling and shrinking the space between the prefilter and the nanopore as its exposed to additional water vapor.
'A remarkable structure'
The device is comprised of three ultrathin layers:
- a nanoporous silicon nitride membrane which serves as a prefilter.
- a biosensor membrane with a single nanopore.
- a spacer layer that separates these by only 200 nm.
The arrangement creates a nanocavity filled with less than a femtoliter of fluid—or about a million times smaller than the smallest raindrops.
During operation, the device uses an electric field to lure a strand of DNA to enter one of the pores of the prefilter and then pass through the nanocavity to reach the pore of the underlying sensor membrane. This triggers changes in the device's electrical current that can be detected and analyzed. The fact that DNA must elongate itself in a consistent way to pass through the two-membrane combination improves the precision and reproducibility of detection.
"This is a remarkable structure," says McGrath. "We've built an integrated system with a highly porous filter within molecular reach of a sensor. I think there are many sensors, particularly those that hunt for biomarkers in raw biological fluids, that would benefit from filtering away unwanted molecules immediately upstream of the detector."
The method of fabrication instantly wets the nanocavity, which is often difficult at the nanoscale. The device contains dozens of these nanocavities, which may eventually increase the amount of material that can be screened by enabling parallelized biomarker detection.
Solving problems that others need solved
Tabard-Cossa's lab uses solid-state nanopore devices to find new ways to manipulate and characterize single molecules. His lab was interested in finding new materials that could be used for biomarker detection. The prefilter in the new device addresses a problem with other silicon nanopore detectors: They are more likely to clog than alternative devices that use that biological pores for sensing. Biological membranes, on the other hand, are less stable than solid state nanopores, McGrath noted.
"We love to apply our membrane technologies to solve problems that others need solved. This is a very nice example.," McGrath says.
McGrath is co-founder of SiMPore, a University-based startup that develops highly portable, chip-based devices that incorporate silicon membranes for a variety of applications, from biological sensing to dialysis.
"I think we're going to realize the practical advantages of this technology in the near term," he says. A second generation of the new device, developed at SiMPore, incorporates the prefilter right on the chips during manufacturing at the wafer scale, "so there's nobody breathing on it anymore," he notes. "It's actually all built as one unit and should make future studies very easy. That's a credit to the ingenuity at SiMPore and quite a legacy for Greg."
In Pursuit of a Universal Flu Vaccine
Friday, November 3, 2017
Study shows pros, cons for major strategy to create broadly protective shot

David J. Topham, Ph.D.
Flu shot season is here. But as you head to the doctor's office or pharmacy to get vaccinated, scientists are working to make this yearly ritual a thing of the past. Researchers around the world, including at the University of Rochester Medical Center (URMC), are pursuing a "universal" flu vaccine, one that would protect against most or all seasonal and pandemic strains of the flu virus.
This is no easy task, and a study out today in the journal Scientific Reports suggests that one of the most promising strategies -- creating a vaccine that targets the "stalk" of a protein that covers the flu virus -- is a strong one, but isn't completely bulletproof.
The hemagglutinin protein, which blankets the outside of the flu virus, looks a bit like a flower; it has a stalk (think stem) and a head (think petals). Current vaccines target the head, which is the part of the virus that's always changing in an effort to evade our immune defenses. The head sits on the stalk, and it's believed that the stalk stays relatively constant from one strain of flu to another. Directing a vaccine and the body's immune response towards the stalk is a seemingly logical strategy for creating a shot that would provide broad protection.
But, contrary to current assumptions, researchers at the URMC-based New York Influenza Center of Excellence (NYICE) found that the stalk can change, although not as easily or frequently as the head.
Using supercomputers at the University's Health Sciences Center for Computational Innovation, they analyzed the genetic sequences of human H1N1 flu viruses circulating since 1918. They found variations in both the head and the stalk, although variability was highest in the head region.
Emma Grygotis wins Outstanding Student Mentor Award
Friday, October 20, 2017

Emma Grygotis, a student in the Cellular and Molecular Pharmacology and Physiology PhD Program was selected by SMD faculty to be this year's recipient of the Outstanding Student Mentor Award. Her selection was based on her contributions to mentoring, leadership, science advocacy, and community outreach.
Emma is currently working in the laboratory of Dr. Denise Hocking, whose laboratory research focuses on understanding the mechanisms by which the extracellular matrix protein, fibronectin, affects cell and tissue functions that are critical for wound repair. Emma's thesis project specifically investigates the mechanisms by which the structure and function of extracellular matrix proteins collagen and fibronectin can be altered by ultrasound for tissue engineering applications.
The award was presented to Emma at the School of Medicine and Dentistry Convocation Ceremony, along with a monetary prize of $500.
Dr. Georas joins the NIH Precision Interventions for Severe and Exacerbation Prone Asthma (PrecISE) Network
Tuesday, October 3, 2017
Dr. Georas has joined the NIH Precision Interventions for Severe and Exacerbation Prone Asthma (PrecISE) Network. This new NIH initiative will establish a network of 10 Clinical Centers in the U.S. conducting innovative research using adaptive clinical trials. Dr. Georas serves as Steering Committee chair, with co-chair Dr. Rosalind Wright.
Minsoo Kim named Dean’s Professorship
Thursday, September 7, 2017

Dr. Minsoo Kim
Dean's Professorships were established in 1982 and are designated by the Dean to be assigned to individuals of outstanding research excellence. Dr. Kim is a Professor of Microbiology and Immunology in the Center for Vaccine Biology and Immunology, and of Pharmacology and Physiology, a position he has held since 2015. He has been a member of the SMD faculty since 2007.
Dr. Kim is an established scientist who has made notable contributions in the field of host immune response against virus infection and cancer. He is especially noted for his pioneering contributions to the development of the most advanced imaging techniques that enable real-time observations of dynamic immune responses. In addition to the real-time "detection" of host immune reaction, Dr. Kim also uses optical technology to actively "control" our immune response against cancer. Cancer immunotherapy is an exciting topic, as it involves stimulating a patient's own immune system to fight the malignancy. Although the concept has been around for 100 years, Dr. Kim is making it more relevant today by using a technology called "optogenetics" (optics + genetics) to try to improve the response rates for immunotherapy.
Join us at the Convocation on September 12th, 2017 (4pm in the Class of '62 Auditorium)
New Patent Issued for Ultrasound Technology
Monday, August 28, 2017
The patent titled "Ultrasound Technology to Control the Spatial Organization of Cells and Proteins in Engineered Tissues" (US 9,688,962) has recently been assigned to the University of Rochester with inventors Diane Dalecki, Ph.D., Denise C. Hocking, Ph.D., and Kelley Garvin, Ph.D. The patent describes novel technology that uses acoustic forces within ultrasound standing wave fields to pattern cells volumetrically and engineer three-dimensional blood vessel networks within hydrogels. Primary applications of this technology include engineering vascularized tissue models for drug testing, tissue engineering, and regenerative medicine. The technology is a result of a multidisciplinary collaboration between faculty members of the Rochester Center for Biomedical Ultrasound, Diane Dalecki (BME) and Denise Hocking (Pharmacology and Physiology) and their former doctoral student Kelley Garvin.
Doctors Might FINALLY Be Able to Tell If Your Infection Is Bacterial or Viral
Monday, August 14, 2017
If you head to your doctor with a fever and cough or other signs you're getting sick, there's a good chance your doctor won't know what's behind it. It's hard to test for certain diseases, like bacterial pneumonia.
When patients come in with respiratory viruses like the common cold and sinus infections, doctors often send them home with an antibiotic prescription. The problem is, antibiotics only target bacteria and won't do anything to fight a virus. Still, about 30 percent of antibiotics prescriptions are for viral infections, according to the CDC. (Make sure to ask these essential questions before taking antibiotics.)
Not only is an antibiotic completely unhelpful against a virus, but it could have major consequences. Antibiotics kill most of the bacteria behind your infection—but not all of them. The bacteria that are strong enough to survive will multiply to create more bacteria that are resistant to treatment, too. Over time, this means there will be more resistant bacteria than ones that antibiotics can kill, so the infections will be harder to treat.
And if you take antibiotics when you don't need to, or use antibacterial soap, you're speeding that process along. Eventually, diseases that used to be easy to treat with antibiotics could become dangerous again.
Luckily, researchers might have found a way to tell the difference between bacterial and viral infections, so you won't get a useless antibiotic. In a study in the journal Scientific Reports, researchers took blood from 94 patients who had lower respiratory tract infections. Lab tests found genetic markers in the blood that could correctly figure out if an infection was viral or bacterial 80 to 90 percent of the time. (Find out how genetic tests could help you lose weight, too.)
Because the sample size was so small, more tests will be needed before doctors can start using this diagnosis method in their offices. But if it does take off, it will be a lot easier than testing for specific diseases.
"Our genes react differently to a virus than they do to bacteria," says study co-author Thomas Mariani, PhD, pediatrics, environmental medicine, and biomedical genetics professor at University of Rochester Medical Center, in a statement. "Rather than trying to detect the specific organism that's making an individual sick, we're using genetic data to help us determine what's affecting the patient and when an antibiotic is appropriate or not."
In the meantime, if you think you're not feeling well, use these natural remedies for cold and flu.
Smyth wins Best Poster award
Thursday, June 22, 2017

Tim Smyth
Congratulations to second year Toxicology graduate student Tim Smyth for winning an award for his poster and presentation at the annual Toxicology Retreat. Tim's poster was entitled "Diesel exhaust particles disrupt epithelial barrier function by altering tricellin expression".
Steve N. Georas Named to New Parkes Professorship
Tuesday, June 13, 2017
 Physician-scientist Steve N. Georas, M.D., professor of Medicine, Environmental Medicine, Microbiology and Immunology, was installed as the inaugural Parkes Family Professor June 5.
Physician-scientist Steve N. Georas, M.D., professor of Medicine, Environmental Medicine, Microbiology and Immunology, was installed as the inaugural Parkes Family Professor June 5.
Walter and the late Carmina Parkes, and their children Susan, Tom and Linda, were driven to open the first asthma center in the region. They have been a steady force in the growth of the UR Medicine's Mary M. Parkes Center for Asthma, Allergy and Pulmonary Care, working closely with center leaders, educators and scientists. The center is located on Red Creek Drive in Henrietta and serves as the leader for the diagnosis, treatment and research of acute asthma, allergies and other pulmonary diseases.
"It was our family's dream to honor the memory of our daughter with the center. Now, establishing a professorship allows us to make it everlasting," said Walter Parkes, chairman of O'Connell Electric Co. The family has committed $1.5 million to the University.
Read the full story.
Papasergi-Scott, Taya, and Wang Win Awards at the GSS Annual Poster Session Competition
Tuesday, May 23, 2017
Congratulations to the following students who won awards at the Graduate Student Society Annual Poster Session Competition held on May 6, 2016.
Makaía M. Papasergi-Scott, working in the laboratories of Dr. Gregory G. Tall and Dr. Robert Freeman, was awarded 1st Place and received a $500 travel reward for her poster titled "Phosphorylation of Ga Chaperone Ric-8A Regulates its Function".
Manisha Taya working in the laboratory of Dr. Stephen R. Hammes, and Xiaowen Wang working in the laboratory of Dr. Mark D. Noble, tied for 3rd place and received $100 travel grants for their posters titled "The Role of Estrogen and Glycoprotein-NMB in Lymphangioleiomyomatosis (LAM) Progression" and "Identifying c-Cbl as a Critical Point of Intervention in Acquired Tamoxifen Resistant Breast Cancer", respectively.
Scientists Light the Way for Immune System to Attack Cancer
Monday, May 15, 2017

The science behind harnessing the immune system to fight cancer is complicated, but a University of Rochester Medical Center laboratory discovered a simple, practical way to use light and optics to steer killer immune cells toward tumors.
In a study published by the online journal Nature Communications, lead author Minsoo Kim, Ph.D., a UR professor of Microbiology and Immunology and a Wilmot Cancer Institute investigator, described his method as similar to "sending light on a spy mission to track down cancer cells."
Immunotherapy is different from radiation or chemotherapy. Instead of directly killing cancer cells, immunotherapy tells the immune system to act in certain ways by stimulating T cells to attack the disease. Several different types of immunotherapy exist or are in development, including pills called "checkpoint inhibitors" and CAR T-cell therapy that involves removing a patient's own immune cells and altering them genetically to seek and destroy cancer cells.
The problem, however, is that immunotherapy can cause the immune system to overreact or under-react, Kim said. In addition, cancer cells are evasive and can hide from killer T-cells. Aggressive tumors also suppress the immune system in the areas surrounding the malignancy (called the microenvironment), keeping T cells out.
Hocking and Roy Receive Patent
Wednesday, February 22, 2017
The patent titled "Chimeric Fibronectin Matrix Mimetics and Uses Thereof" (U.S. Patent No. 9,572,869; awarded February 21, 2017) has recently been assigned to the UR with inventors Denise Hocking, Ph.D. and Daniel Roy, Ph.D. (BME B.S.'06, Ph.D.'12). The patent relates to the use of recombinant fibronectin-based peptides for wound healing and tissue regeneration applications. The technology falls under a new and exciting class of therapies known as wound biologics. The primary commercial application for this technology is to promote healing of hard-to-heal or chronic wounds, including diabetic, venous, and pressure ulcers, which impose a significant health care burden worldwide. Topical application of fibronectin matrix mimetic peptides to full-thickness excisional wounds in diabetic mice accelerates wound closure and promotes granulation tissue deposition, remodeling, and re-vascularization.
Denise Hocking, PhD is a Professor of Pharmacology and Physiology and of Biomedical Engineering. Daniel Roy is a Scientist at KeraNetics, LLC, a biotechnology company located in Winston-Salem, North Carolina that develops keratin-based biomaterials for wound healing applications.