News
New Research Findings Published in 2020 from Yao Lab
Wednesday, December 30, 2020
Cardiovascular disease (CVD is the number one killer of human beings and have recently resulted even more human death in combination with COVID19 virus infection during the pandemic. As exemplified by the two recently FDA approved mRNA vaccines from Pfizer and Moderna companies, we are so excited that mRNA plus translation can make our own cells manufacture vaccine proteins to protect us from virus infections and relieve the burden for our cardiovascular system. Importantly, mRNAs can also produce therapeutic proteins via the translation process to fight against a variety of diseases including cardiovascular disease and cancer. Our research perfectly aligns with the goal and missions of these pharmaceutical companies in aiming to utilize or manipulate mRNA translation to treat human diseases.
In July 2020, we published our results in Circulation Research [1] about the function of an evolutionarily conserved, house-keeping enzyme glutamyl-prolyl-tRNA synthetase in cardiac fibrosis, and a Chinese medicine derived compound halofuginone in antagonizing fibrosis via inhibiting proline-rich protein translation. This compound was used for treating malaria infection and arrhythmia in ancient China for more than 2000 years. High dose of the drug can inhibit global protein synthesis via blocking the incorporation of proline amino acid, a building block of mRNA translation. Our data suggests that low dose of this drug actually can provide therapeutic benefit in heart disease treatment by mildly inhibiting translation of proline-rich proteins, which are mostly encoded by pro-fibrotic extracellular matrix transcripts in cardiac fibroblasts. Selective repression of expression of these fibrogenic proteins by knocking out one allele of Eprs gene in loss-of-function genetic mouse models or inhibiting enzymatic activity of EPRS protein, remarkably reduces cardiac fibrosis and hypertrophy, and partially restored cardiac function in heart failure mouse models.
On December 28th 2020, we had another paper published online in EMBO Molecular Medicine [2] showing that a cardioprotective small noncoding RNA, microRNA-574 (miR-574), plays important role in maintaining mitochondrial translation homeostasis and normal cardiac function via fine-tuning the expression of a novel mitochondrial translation regulatory factor FAM210A (family with sequence similarity 210 member A). Mitochondria are the power houses of living cells. They provide ATP as energy currency to support any biochemical reactions in our own body. Dysfunction of mitochondria caused by aberrant expression of mitochondrial proteins is known to trigger severe mitochondrial cardiomyopathy and heart failure. In this work, we used an miR-574 genetic knockout mouse model and transcriptome-wide gene expression profiling to discover that miR-574-FAM210A axis plays a critical role in maintaining optimal mitochondrial protein translation and protecting heart functions under cardiac stress conditions.
To confirm the relevance of EPRS and miR-574 in human heart failure, we collaborated with a cardiologist and physician scientist Dr. Wai Hong Wilson Tang from Cleveland Clinic to obtain human failing heart and non-failure tissue samples to measure the expression of EPRS and miR-574 and validated their relationship with human heart disease. A major challenge in these studies is to define the downstream molecular targets of EPRS and miR-574. With the extensive collaborative efforts from the University of Rochester Genomics Core, we performed the polysome profiling coupled with next generation RNA deep sequencing for halofuginone-treated, EPRS-inhibited fibroblast cells, and RNA-sequencing for miR-574 knockout mouse hearts, respectively. We successfully identified the target mRNAs of EPRS and miR-574 and discovered a number of novel therapeutic targets (such as SULF1 and FAM210A, respectively) for treatment of cardiac fibrosis and pathological remodeling.
- Jiangbin Wu, Kadiam C Venkata Subbaiah, Li Huitong Xie, Feng Jiang, Eng-Soon Khor, Deanne Mickelsen, Jason R. Myers, Wai Hong Wilson Tang, and Peng Yao. Glutamyl-prolyl-tRNA synthetase regulates proline-rich pro-fibrotic protein synthesis during cardiac fibrosis. Circulation Research 2020 127:827–846
- Jiangbin Wu, Kadiam C Venkata Subbaiah, Feng Jiang, Omar Hedaya, Amy Mohan, Tingting Yang, Kevin Welle, Sina Ghaemmaghami, Wai Hong Wilson Tang, Eric Small, Chen Yan, and Peng Yao. MicroRNA-574 regulates FAM210A expression and influences pathological cardiac remodeling. EMBO Molecular Medicine Online published (https://doi.org/10.15252/emmm.202012710)
Blog: Are the new mRNA vaccines safe?
Wednesday, December 30, 2020

John Lueck
Joanne Ladolcetta, a freelance writer based out of San Francisco, wanted to put together information to help address circulating fears and misconceptions about the recently FDA approved COVID-19 mRNA vaccines. She reached out to friends Bryant Johnson (Cartoonist) and John Lueck (Researcher at the University of Rochester Medical Center) to tackle some of the most common vaccine questions. The collaboration resulted in an approachable informative write-up with creative illustrations and helpful links to provide readers the ability to do a deeper dive into the generation and application of the novel COVID-19 mRNA vaccines.

Courtesy of Bryant Johnson
University of Rochester Center for RNA Biology Presentation Contest, January 2021
Wednesday, December 23, 2020
RNA Presentations by UR Grad Students, Postdocs, and Techs!
Sponsored by the RNA Society, Lexogen, and the UR Center for RNA Biology
Virtual presentations will be on Wednesdays at 2pm EST on January 6th, 13th, & 27th during the usual Department of Biochemistry & Biophysics Seminar. There will be 3-4 presentations on each date (~20-min slots). Winners will be determined by the anonymous panel of judges based on the quality of their lay and research presentations and their ability to address questions during the Q&A session.
JANUARY 2021 PRESENTATION SCHEDULE
Day 1: Wed, Jan 6th
2:00 -- 3:20pm
|
2:00pm |
Lily Cisco, Lueck lab, Pharm-Phys grad student |
|
2:20pm |
Xueyang He, Boutz lab, Biophysics grad student |
|
2:40pm |
Shon Koren, Johnson lab, technician |
|
3:00pm |
Kamel Awayda, O'Connell lab, technician |
Day 2: Wed, Jan 13th
2:00 -- 3:00pm
|
2:00pm |
Chen Bao, Ermolenko lab, Biochemistry grad student |
|
2:20pm |
Sean Lindley, Anderson lab, Biology grad student |
|
2:40pm |
Arica VanderWal, O'Connell lab, Biochemistry grad student |
Day 3: Wed, Jan 27th
2:00 -- 3:00pm
|
2:00pm |
Deb Maji, Kielkopf lab, Biophysics grad student |
|
2:20pm |
Elizabeth Abshire, PhD, Maquat lab, postdoc |
|
2:40pm |
Xavier Rambout, PhD, Maquat lab, postdoc |
Contestants for oral presentations were selected based on the scientific merit of their abstract submissions, in December, by an anonymous panel of six faculty judges and two graduate student judges. Competitors will present a 2-min lay talk without slides followed by a 12-min slide presentation of their research, i.e. the topic of their abstract, and a 3-min Q&A session.
COVID-19 vaccine: What’s RNA research got to do with it?
Monday, December 14, 2020
The US Food and Drug Administration recently approved emergency use authorization for a COVID-19 vaccine developed by Pfizer and the German pharmaceutical company BioNTech.
The vaccine made history not only because it reported a 95 percent efficacy rate at preventing COVID-19 in clinical trials, but because it is the first vaccine ever approved by the FDA for human use that is based on RNA technology.
"The development of RNA vaccines is a great boon to the future of treating infectious diseases," says Lynne Maquat, the J. Lowell Orbison Distinguished Service Alumni Professor in biochemistry and biophysics, oncology, and pediatrics at Rochester and the director of Rochester's Center for RNA Biology.
COVID-19, short for "coronavirus disease 2019," is caused by the novel coronavirus SARS-CoV-2. Like many other viruses, SARS-CoV-2 is an RNA virus. This means that, unlike in humans and other mammals, the genetic material for SARS-CoV-2 is encoded in ribonucleic acid (RNA). The viral RNA is sneaky: its features cause the protein synthesis machinery in humans to mistake it for RNA produced by our own DNA.
For that reason, several of the leading COVID-19 vaccines and treatments are based on RNA technology.
A contingent of researchers at the University of Rochester study the RNA of viruses to better understand how RNAs work and how they are involved in diseases. This RNA research provides an important foundation for developing vaccines and other drugs and therapeutics to disrupt the virus and stop infections.
"Understanding RNA structure and function helps us understand how to throw a therapeutic wrench into what the COVID-19 RNA does—make new virus that can infect more of our cells and also the cells of other human beings," Maquat says.
In the past few decades, as scientists came to realize that genetic material is largely regulated by the RNA it encodes, that most of our DNA produces RNA, and that RNA is not only a target but also a tool for disease therapies, "the RNA research world has exploded," Maquat says. "The University of Rochester understood this."
In 2007, Maquat founded The Center for RNA Biology as a means of conducting interdisciplinary research in the function, structure, and processing of RNAs. The Center involves researchers from both the River Campus and the Medical Center, combining expertise in biology, chemistry, engineering, neurology, and pharmacology.
Cool Technology Allows for Better Views of Cancerous Blood Cells in Quest for New Treatment
Tuesday, September 15, 2020

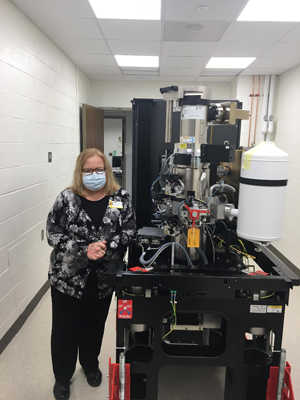
Clara Kielkopf, Ph.D., left, and Laura Calvi, M.D., stand by the University's new cryo-microscope
With the recent acquisition of Nobel Prize-winning technology and two new grants, Wilmot Cancer Institute researchers are streamlining their investigations into a malignant blood disease known as MDS, working toward discovering targeted therapies.
Laura Calvi, M.D., and Clara Kielkopf, Ph.D., are leading collaborative teams that will be using a device at the University of Rochester Medical Center — a cryo-electron microscope — that has ushered in a new era in biochemistry. The microscope allows scientists to see 3D snapshots and more details of living molecules than ever before, down to near-atomic resolution, to understand disease and uncover new ways to design drugs. The developers of the technology were awarded the Nobel Prize in Chemistry 2017.
Calvi and Kielkopf each received Edward P. Evans Foundation awards totaling $1.2 million for this project. Evans grants go to scientists seeking a cure for myelodysplastic syndromes (MDS), which originates in the bone marrow and disrupts healthy blood cell formation. MDS often leads to leukemia.
The Kielkopf lab will use the cryo-electron microscope to obtain 3D views of recurrent MDS mutations as guides for targeting molecular therapies. The modern microscope is the first of its kind in the Rochester region, Kielkopf said, and will be accessible to all UR researchers through the Electron Microscopy Shared Resource Laboratory. She is a professor of Biochemistry and Biophysics in the Center for RNA Biology.
David Mathews, MD, PhD installed as first Lynne Maquat Distinguished Professor in RNA Biology
Tuesday, September 15, 2020
We are thrilled to announce that Professor David Mathews, MD, PhD, was honored at this year's School of Medicine Opening Convocation by being installed as the first Lynne Maquat Distinguished Professor in RNA Biology in the School of Medicine and Dentistry. The professorship was awarded by Dr. Mark Taubman, Dean of the School of Medicine and CEO of the University of Rochester Medical Center. The Convocation was held virtually this year and can be viewed at the link below.
Congratulations to Professor Mathews!
RNA Essay Contest Results and Congratulations
Wednesday, August 26, 2020
The UR Center for RNA Biology offered an exercise during the time when COVID-19 became a sufficient threat to largely shut-down our research enterprise. We're pleased to announce the winners of the UR's Center for RNA Biology Essay Contest on "The role of RNA research in community health". These awards are sponsored by a grant from the RNA Society & Lexogen to the UR Center for RNA Biology, and funds from UR RNA Structure & Function Cluster.
Our Gold prize (~$1,000 value) award goes to Sydney Simpson, an Immunology, Microbiology & Virology graduate student in Steve Dewhurst's lab in the Department of Microbiology & Immunology, for her essay: "Nucleoside Analog Inhibitors: Timeless & Timely Beacons of Hope".
The Silver Prize (~$250 value) award goes to Omar Hedaya, a Biochemistry & Molecular Biology graduate student in Peng Yao's lab in the Department of Medicine/Department of Biochemistry & Biophysics, for his essay: "Know the Fundamentals when Seeking the Future".

Omar Hedaya

Sydney Simpson
Both awardees have become members of the RNA Society and will use their winnings toward technology needs for the upcoming semester.
The RNA Society now features our contest results, including the winning essays, in its latest RNA Salon update: https://www.rnasociety.org/featured-salons, see bullet #3.
We would like to acknowledge Honorable Mentions for the following applicants:
- Sai Shashank Chavali -- Graduate student; Biophysics, Structural & Computational Biology; Wedekind Lab
- Lily Cisco -- Graduate student; Cellular and Molecular Pharmacology & Physiology; Lueck Lab
- Gabrielle Kosoy -- Graduate student; Biophysics, Structural & Computational Biology; Miller Lab
- Ashwin Kumar -- Graduate student; Biophysics, Structural & Computational Biology; Topham Lab
- Li Xie -- Graduate student; Genetics, Development & Stem Cells; Pröschel Lab
We thank all who participated -- and our judges, too!
Kielkopf Lab Awarded EvansMDS Research Grant
Thursday, July 16, 2020

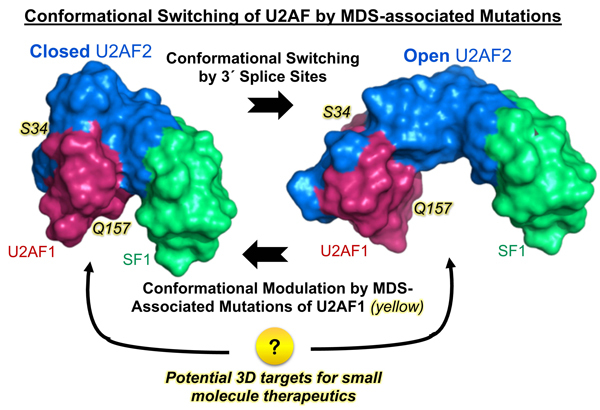
Prof. Clara Kielkopf has been awarded an EvansMDS research grant to use the new, state-of-the-art cryo-electron microscope at the U of R Medical Center EM facility. The Kielkopf group will use this revolutionary technique to study 3D structures of mutant U2AF1-splicing complexes in human malignancies. The title of the EvansMDS grant is: "Cryo-Electron Microscopy Structures of Mutant U2AF1-Containing Ribonucleoproteins Associated with Myelodysplastic Syndromes". This grant was made possible by our new Talos cryo-EM.
This week’s URMC Research Heroes featured the Maquat lab’s Tatsuaki Kurosaki, PhD, and Shuhei Mitsutomi, MS
Wednesday, June 3, 2020

This week's URMC Research Heroes featured the Maquat lab's Tatsuaki Kurosaki, PhD, and Shuhei Mitsutomi, MS, who were recognized today for their work on SARS-CoV-2.
https://www.instagram.com/p/CA-QN7oAl07/
Both Tatsuaki and Shuhei have worked as members of the Maquat Lab (Center for RNA Biology and the Department of Biochemistry & Biophysics) during the sequestration on SARS-CoV-2, collaborating with a lab at Harvard to determine the mechanism by which the virus inhibits human-cell nonsense-mediated mRNA decay (NMD) so as to express and replicate its RNA efficiency.
From Tatsuaki: "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease 19 (COVID-19) pandemic, is a novel enveloped RNA virus carrying a large (~30 kb) positive-sense single-stranded RNA genome. Although human cells innately have an RNA surveillance pathway called nonsense-mediated mRNA decay (NMD) that generally protects cells from infection by many different types of viruses, little is known about how SARS-CoV-2 inhibits NMD to proliferate in human cells. We hope that our research helps to elucidate the molecular mechanisms of SARS-CoV-2 proliferation in human cells, eventually contributing toward the development of therapeutic strategies to combat COVID-19."
University of Rochester RNA Essay Contest: “The role of RNA research in community health”
Wednesday, May 20, 2020

Sponsored by the RNA Society & Lexogen, UR RNA Structure & Function Cluster, and UR Center for RNA Biology
Who is eligible: Any University of Rochester graduate student or post-doc with an interest in RNA biology
Entry rules: Essays should be no more than two pages, single-spaced (excluding references, which should be present), 11-point Arial font, with half-inch margins all around.
Prizes: Two prizes will be given out. Gold (valued at ~$1000), and Silver (valued at ~$250). Additionally, winning essays along with photos of the winning authors will be posted on the Center for RNA Biology webpage and featured on the RNA Society's RNA Salon page, offering international exposure.
Details
The UR's Center for RNA Biology is running an essay contest, sponsored by the RNA Society & Lexogen, and UR's RNA Structure & Function Cluster, on "The role of RNA research in community health". This contest, which is open to all UR graduate students and post-docs, aims to promote creative yet data-driven thinking about the importance of RNA in the "big picture".
Considering that reliable technology is required for research in an increasingly virtual world, prizes will consist of a PC or Mac laptop for Gold winners (~$1000), and software licenses or peripherals (e.g., second monitor or laptop dock) for Silver winners (~$250), subject to the needs of each recipient.
Submissions must be emailed to Liz by Monday, July 13th, 2020.
Winners will be announced in the beginning of August.
Links
The RNA Society: https://www.rnasociety.org/
RNA Structure & Function Cluster: http://www.rochester.edu/ucis/rnastructure.html
Center for RNA Biology: https://www.urmc.rochester.edu/rna-biology.aspx
Center for RNA Biology Contributes to Fighting Coronavirus
Tuesday, April 28, 2020
Viruses like the coronavirus that causes COVID-19 are able to unleash their fury because of a devious weapon: ribonucleic acid, also known as RNA.
A contingent of researchers at the University of Rochester study the RNA of viruses to better understand how RNAs work and how they are involved in diseases. As COVID-19 continues to spread around the globe, RNA research provides an important foundation for developing antiviral drugs, vaccines, and other therapeutics to disrupt the virus and stop infections.
"Understanding RNA structure and function helps us understand how to throw a therapeutic wrench into what the COVID-19 RNA does—make new virus that can infect more of our cells and also the cells of other human beings," says Lynne Maquat, professor of biochemistry and biophysics at the University of Rochester Medical Center and the director of Rochester's Center for RNA Biology.
In the past few decades, as scientists came to realize that genetic material is largely regulated by the RNA it encodes, that most of our DNA produces RNA, and that RNA is not only a target but also a tool for disease therapies, "the RNA research world has exploded," Maquat says. "The University of Rochester understood this."
In 2007, Maquat founded the Center for RNA Biology as a means of conducting interdisciplinary research in the function, structure, and processing of RNAs. The center involves researchers from both the River Campus and the Medical Center, combining expertise in biology, chemistry, engineering, neurology, and pharmacology.
While much of the research across the University has been put on pause, labs that are involved in coronavirus research remain active.
"Our strength as a university is our diversity of research expertise, combined with our highly collaborative nature," says Dragony Fu, an assistant professor of biology on the River Campus and a member of the Center for RNA Biology. "We are surrounded by outstanding researchers who enhance our understanding of RNA biology, and a medical center that provides a translational aspect where the knowledge gained from RNA biology can be applied for therapeutics."