News
URMC-099 Combats Surgery-Induced Delirium, Cognitive Dysfunction in Preclinical Model of Orthopedic Surgery
Wednesday, November 6, 2019
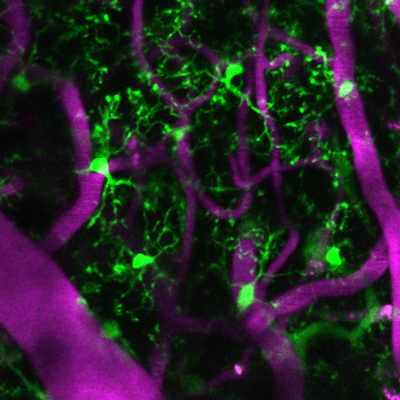
Living microglia, genetically marked to glow green, in the cerebral cortex with magenta colored blood vessels from a mouse treated with URMC-099.
A new study published in the Journal of Neuroinflammation found that prophylactic treatment with URMC-099 -- a "broad spectrum" mixed-lineage kinase 3 inhibitor -- prevents neuroinflammation-associated cognitive impairment in a mouse model of orthopedic surgery-induced perioperative neurocognitive disorders (PND).
PND, a new term that encompasses postoperative delirium, delayed neurocognitive recovery, and postoperative neurocognitive disorder, is the most common complication after routine surgical procedures, particularly in the elderly. Following surgery, such as hip replacement or fracture repair, up to 50 percent of patients experience cognitive disturbances like anxiety, irritability, hallucinations, or panic attacks, which can lead to more serious complications down the line. Currently, there are no FDA-approved therapies to treat it.
Developed in the laboratory of Harris A. "Handy" Gelbard, M.D., Ph.D., director of the Center for Neurotherapeutics Discovery at the University of Rochester Medical Center, URMC-099 inhibits damaging innate immune responses that lead to inflammation in the brain and accompanying cognitive problems. Using animal models of diseases like HIV-1-associated neurocognitive disorders, Alzheimer's disease and multiple sclerosis, Gelbard has shown that the compound blocks enzymes called kinases (such as mixed lineage kinase type 3, or MLK3) that respond to inflammatory stressors inside and outside cells.
Gelbard and Niccolò Terrando, Ph.D., director of the Neuroinflammation and Cognitive Outcomes laboratory in the Department of Anesthesiology at Duke University Medical Center, used an orthopedic surgery mouse model that recapitulates features of clinical procedures such as a fracture repair or hip replacement, which are often associated with PND in frail subjects. In a pilot experiment, they treated one group of these mice with URMC-099 before and after surgery, and another group prior to surgery only. Gelbard and Terrando's teams, including first author Patrick Miller-Rhodes, a senior pre-doctoral student in the Neuroscience Graduate Program working in the Gelbard lab at URMC, measured the following:
- How the surgery affected the central nervous system and the immune cells (microglia) that reside there was evaluated using stereology and microscopy.
- Surgery-induced memory impairment was assessed using the "What-Where-When" and Memory Load Object Discrimination tasks.
- The acute peripheral immune response to surgery was assessed by cytokine/chemokine profiling and flow cytometry.
- Long-term fracture healing was assessed in fracture callouses using micro-computerized tomography and histomorphometry analyses.
- For additional details see the "Materials and Methods" section of the study
The team found that the surgery disrupted the blood brain barrier and activated microglia (a first line immune responder present in the inflamed brain), which led to impaired object place and identity discrimination when the mice were subject to the "What-Where-When" and Memory Load Object Discrimination tasks. Both URMC-099 dosing methods prevented the surgery-induced microgliosis (increase in the number of activated microglia) and cognitive impairment without affecting fracture healing.
"A major concern regarding the use of anti-inflammatory drugs for PND is whether they will affect fracture healing. We found that our preventive, time-limited treatment with URMC-099 didn't influence bone healing or long-term bone repair," said Gelbard and Terrando, professor of Neurology, Neuroscience, Microbiology and Immunology, and Pediatrics at URMC and associate professor of Anesthesiology at Duke University Medical Center, respectively. "These findings of improvement in cognition and normal fracture healing provide compelling evidence for the advancement of URMC-099 as a therapeutic option for PND."
"Right now we have nothing to treat this condition," said Mark A. Oldham, M.D., assistant professor in the department of Psychiatry at URMC who treats patients with PND. "We work hard to provide good medical care, including helping people sleep at night and making sure they are walking, eating and drinking, but it isn't clear that these efforts have any meaningful long-term impact."
According to Oldham, recent studies that track patients following an episode of PND show that many of them don't resolve completely, and that they have a new cognitive baseline after delirium.
"It is increasingly an accepted fact that after delirium, people have suffered some kind of neurological insult, which leaves them cognitively or functionally worse off than before the incident," he noted.
Next steps for the research include identifying definitive mechanisms for pain modulation, immune cell trafficking and neuro-immune characterization in PND. Gelbard and Terrando are tackling some of these questions with funds from the National Institutes of Health (RO1 AG057525). The current study was also funded by multiple grants from the NIH (P01MH64570, RO1 MH104147, RO1 AG057525 and F31 MH113504). The University of Rochester has four issued U.S. patents and multiple issued patents in foreign countries covering URMC-099.
Neuroscience Students and Faculty Receive Awards at Convocation
Monday, September 23, 2019
Neuroscience students and faculty made a big splash at the 2019 School of Medicine & Dentistry Convocation Ceremony. The following were awarded and recognized for their achievements:
- Martha Gdowski, Ph.D., received the Manuel D. Goldman Prize for Excellence in First Year Teaching & the Marvin J. Hoffman Faculty Mentoring Award
- John Olschowka, Ph.D., received a Commendation for First Year Teaching
- Sergiy Nadtochiy, Ph.D., received a Commendation for First Year Teaching
- Sarah McConnell, Ph.D., received the Gold Medal Award for Excellence in Teaching
- Johanna Fritzinger, first year NGP student, received the Irving L. Spar Fellowship Award
- Sarah Yablonski, first year NGP student, received the Merritt & Marjorie Cleveland Fellowship & the Robert L. and Mary L. Sproull University Fellowship
- Suzanne Haber, Ph.D., received the Dean's Professorship
- Marc Halterman, M.D., received the Dean's Associate Professorship
Congratulations to all recipients!
Professor Maddox receives funding from NIH and NIDCD
Friday, August 23, 2019
Professor Ross Maddox has received funding from the National Institutes of Health (NIH) and the National Institute on Deafness and Other Communication Disorders (NIDCD) for his project, "Rapid acquisition of the frequency-specific auditory brainstem response through parallel stimulus presentation." The frequency-specific auditory brainstem response (ABR) is a diagnostic tool that is used to test for hearing loss in infants and prescribe treatments such as behavioral intervention or hearing aids. The ABR is performed while the baby sleeps, which leads to tests with unpredictable durations that are often too short to provide complete information. This project is relevant to public health because it provides a new kind of ABR exam that can be run much faster, allowing more complete and accurate diagnoses to be made.
NGP Student Article Published in the bioRxiv
Monday, August 19, 2019
Abstract: Perioperative neurocognitive disorders (PND), including delirium and postoperative cognitive dysfunction, are serious complications that afflict up to 50% of surgical patients and for which there are no disease-modifying therapeutic options. Here, we test whether prophylactic treatment with the broad spectrum mixed-lineage kinase 3 inhibitor URMC-099 prevents surgery-induced neuroinflammation and cognitive impairment in a translational model orthopedic surgery-induced PND. We used a combination of two-photon scanning laser microscopy and CLARITY with light-sheet microscopy to define surgery-induced changes in microglial morphology and dynamics. Orthopedic surgery induced microglial activation in the hippocampus and cortex that accompanied impairments in episodic memory. URMC-099 prophylaxis prevented these neuropathological sequelae without impacting bone fracture healing. Together, these findings provide compelling evidence for the advancement of URMC-099 as a therapeutic option for PND.
Professor Carney receives renewed NIH funding
Monday, July 15, 2019
Congratulations to Professor Laurel Carney on the renewal of her NIH R01 for her project, "Auditory Processing of Complex Sounds." The Carney Lab hypothesizes that midbrain sensitivity to neural amplitude and frequency fluctuations in peripheral responses provides a robust representation of complex sounds, including speech. Aim 1 tests this hypothesis with physiological and behavioral studies of midbrain responses to stimuli that combine these cues, including "designer" stimuli with conflicting cues to determine how they may interact. These results will be used to test and refine a new computational model for midbrain responses with sensitivity to these cues. Aim 2 tests the hypothesis with physiological responses of midbrain neurons to voiced speech, to directly test model predictions based on characterization of each neuron's sensitivity to these cues. Understanding the role in speech coding of the amplitude and frequency fluctuations in peripheral responses is clinically significant because these fluctuations are vulnerable to SNHL. In Aim 3, we will test the hypothesis that amplitude and frequency fluctuations can be manipulated in synthetic speech to influence intelligibility in human listeners with or without SNHL, in quiet and in noise. Preliminary results from modeling, physiological, and behavioral studies support the proposed hypotheses. Ultimately, our goal is to extend this approach to manipulate fluctuation contrasts in running speech, to effectively "correct" sound for the impaired ear.
Brain stimulation speeds up visual learning in healthy adults, helps patients re-learn how to see
Tuesday, May 28, 2019
Practice results in better learning. Consider learning a musical instrument, for example: the more one practices, the better one will be able to learn to play. The same holds true for cognition and visual perception: with practice, a person can learn to see better—and this is the case for both healthy adults and patients who experience vision loss because of a traumatic brain injury or stroke.
The problem with learning, however, is that it often takes a lot of training. Finding the time can be especially difficult for patients with brain injuries who may, for instance, need to re-train their brains to learn to process visual cues.
But what if this learning process could be accelerated?
That's what University of Rochester researchers Duje Tadin, a professor of brain and cognitive sciences, and Krystel Huxlin, the James V. Aquavella, M.D. Professor in Ophthalmology at the University's Flaum Eye Institute, set out to determine. Motivated by emerging evidence that brain stimulation might aid learning, Tadin and Huxlin collaborated with researchers at the Italian Institute of Technology to study how different types of non-invasive brain stimulation affect visual perceptual learning and retention in both healthy individuals and those with brain damage. Their results, published in a paper in the Journal of Neuroscience, could lead to enhanced learning efficacy for both populations and improved vision recovery for cortically blind patients.
Enhancing learning with brain stimulation
Learning is difficult and often takes a long time, Tadin says, "because after early childhood our brains become less plastic." The brain's ability to change and reorganize itself decreases as a person ages, so learning new tasks, or re-learning tasks after experiencing a brain injury, becomes more challenging.
To test if and how visual perceptual learning might be accelerated, researchers presented study participants with a computer-based task. Participants were shown clouds of dots and were asked to determine which way the dots moved across the computer screen. The task measured the participants' motion integration threshold; motion perception is important in enabling people to see movement and either to avoid or interact with moving objects. Participants were then asked to perform the task while sub-groups were given different types of brain stimulation, each involving a non-invasive electrical current applied over the visual cortex. The researchers found that one particular type of brain stimulation, called transcranial random noise stimulation (tRNS), had remarkable effects on improving participants' motion integration thresholds when they performed the task.
"All groups of participants got better at the dot motion task with practice, but the group that also trained with tRNS improved twice as much and was able to learn the motion task better than other groups," Tadin says.
Surprisingly, the researchers also found that when they re-tested the participants six months later, the boosts in performance were still there: the participants in the tRNS group had retained what they had learned and were still able to do better on the motion task compared to the groups that were given other stimulation techniques or training alone.
In The News: Interviews with Scientists: Rianne Stowell
Tuesday, May 7, 2019
 Rianne Stowell, fifth year graduate student in the Majewska Lab, is interviewed by Hello Bio about her research, the most important lesson she's learned as Ph.D. student, her proudest career moment and more.
Rianne Stowell, fifth year graduate student in the Majewska Lab, is interviewed by Hello Bio about her research, the most important lesson she's learned as Ph.D. student, her proudest career moment and more.
Dr. Gary Paige is awarded 2019 Peter Shrager Award
Friday, May 3, 2019
 Dr. Gary Paige is the 2019 recipient of the Peter Shrager Award, which recognizes exceptional service to the Rochester neuroscience community. The award was established in 2017 to honor Dr. Peter Shrager, professor in the Department of Neuroscience, for his commitment to the education and research mission of the neuroscience enterprise at the University over his last 40 years as a faculty member. Dr. Gary Paige served as the chair of Neurobiology and Anatomy for more than ten years and was a tireless advocate for neuroscience faculty and students. In this role, he built a very open neuroscience community, expanding the department through secondary appointments and encouraging faculty be active members of the neuroscience community at large. He was a strong supporter of graduate and medical education, using creative solutions to forge collaborative and interdisciplinary research that integrated basic, translational and clinical approaches. Since stepping down as chair, he has continued to be influential in creative ways by marrying his two loves—art and neuroscience—in the Arts in Mind project, an educational experience for UR students and faculty. Dr. John Foxe presented the award at the annual neuroscience retreat on Friday, April 12.
Dr. Gary Paige is the 2019 recipient of the Peter Shrager Award, which recognizes exceptional service to the Rochester neuroscience community. The award was established in 2017 to honor Dr. Peter Shrager, professor in the Department of Neuroscience, for his commitment to the education and research mission of the neuroscience enterprise at the University over his last 40 years as a faculty member. Dr. Gary Paige served as the chair of Neurobiology and Anatomy for more than ten years and was a tireless advocate for neuroscience faculty and students. In this role, he built a very open neuroscience community, expanding the department through secondary appointments and encouraging faculty be active members of the neuroscience community at large. He was a strong supporter of graduate and medical education, using creative solutions to forge collaborative and interdisciplinary research that integrated basic, translational and clinical approaches. Since stepping down as chair, he has continued to be influential in creative ways by marrying his two loves—art and neuroscience—in the Arts in Mind project, an educational experience for UR students and faculty. Dr. John Foxe presented the award at the annual neuroscience retreat on Friday, April 12.
NGP Alum, Aleta Steevens, awarded Doty prize
Friday, May 3, 2019

Aleta Steevens, recent doctoral graduate from the Kiernan lab, received the Robert Doty prize for the 2019 outstanding dissertation in neuroscience. The Doty prize is named in the honor of longtime faculty member Robert Doty, who made great contributions to neuroscience research at the University of Rochester and nationally. It is awarded on the basis of the impact and importance of research, novelty of experimental design, independence and creativity of the student and research implications and relevance for neuroscience. Aleta's thesis entitled "The Dynamic Role of SOX2 in Mammalian Inner Ear Development," which she successfully defended on April 16, 2018, embodied all these characteristics. Aleta was also awarded an NIH predoctoral fellowship, and her work has resulted in two first-author publications. Beyond her research, Aleta was exceptionally active and successful in teaching. She served as a TA in the course Biology of Mental Disorders, and won the Edward Peck Curtis teaching award in 2016. Aleta has now moved to her postdoctoral position with Dr. Walter Low at the University of Minnesota. Dr. Peter Shrager presented the prize at the annual neuroscience retreat on Friday, April 12.
Brendan Whitelaw receives 2019 Edward Peck Curtis Awards for Excellence in Teaching by a Graduate Student
Tuesday, April 16, 2019
 Neuroscience Graduate Program and Medical Scientist Training Program student Brendan Whitelaw has been selected as one of the recipients of the 2019 Edward Peck Curtis Awards for Excellence in Teaching by a Graduate Student. The awards were established to recognize graduate students who advance the teaching mission of the University by providing highly skilled and innovative undergraduate education. Students were nominated by their department chair and a faculty member. Congratulations, Brendan!
Neuroscience Graduate Program and Medical Scientist Training Program student Brendan Whitelaw has been selected as one of the recipients of the 2019 Edward Peck Curtis Awards for Excellence in Teaching by a Graduate Student. The awards were established to recognize graduate students who advance the teaching mission of the University by providing highly skilled and innovative undergraduate education. Students were nominated by their department chair and a faculty member. Congratulations, Brendan!
Become a Member of the Del Monte Institute for Neuroscience
Tuesday, March 5, 2019
The Del Monte Institute for Neuroscience brings together faculty across the University of Rochester who are engaged in neuroscience research.
Members will receive exclusive benefits for belonging to the Del Monte network:
- Eligibility to apply and receive funding for pilot projects, or other activities that support Institute goals
- Print and email subscription to our quarterly publication, Neuroscience
- Opportunities to volunteer for community outreach activities
- Access to Institute listservs to promote relevant activities
- Listing on the Institute website, using information from your University profile to facilitate networking and visibility
You must be a faculty member or postdoctoral fellow at the University of Rochester with an interest in brain science to become a member. Click here to apply.
Neuroscience Pilot Grants Available
Tuesday, March 5, 2019
The Ernest J. Del Monte Institute for Neuroscience is pleased to announce the availability of up to 21 pilot project awards (maximum budget of $50,000 per award) to support novel basic, clinical and translational projects in the neurosciences. These awards will be supported under five programs for 2019 and are open to all faculty members across both the Medical School and the Undergraduate Campus. Funds available for this year's program are $840,000.
The application deadline is 5:00 pmon Monday, March 18, 2019.
Not All Sleep is Equal When It Comes to Cleaning the Brain
Wednesday, February 27, 2019
New research shows how the depth of sleep can impact our brain's ability to efficiently wash away waste and toxic proteins. Because sleep often becomes increasingly lighter and more disrupted as we become older, the study reinforces and potentially explains the links between aging, sleep deprivation, and heightened risk for Alzheimer's disease.
"Sleep is critical to the function of the brain's waste removal system and this study shows that the deeper the sleep the better," said Maiken Nedergaard, M.D., D.M.Sc., co-director of the Center for Translational Neuromedicine at the University of Rochester Medical Center (URMC) and lead author of the study. "These findings also add to the increasingly clear evidence that quality of sleep or sleep deprivation can predict the onset of Alzheimer's and dementia."
The study, which appears in the journal Science Advances, indicates that the slow and steady brain and cardiopulmonary activity associated with deep non-REM sleep are optimal for the function of the glymphatic system, the brain's unique process of removing waste. The findings may also explain why some forms of anesthesia can lead to cognitive impairment in older adults.
Grant Marks Two Decades of NIH Support for Muscular Dystrophy Research
Monday, February 25, 2019
The University of Rochester Medical Center (URMC) has received $8 million from the National Institutes of Health (NIH) to support pioneering research on muscular dystrophy. The grant, which is a renewal of URMC's Paul D. Wellstone Muscular Dystrophy Cooperative Research Center, will fund ongoing work to investigate the genetic mechanisms and progression of this complex multi-system disease, research that has led scientists to the threshold of potential new therapies for myotonic dystrophy.
Neuroscience Professor and Alumna Inducted to Alpha Omega Alpha
Wednesday, February 13, 2019
Congratulations to Neuroscience associate professor Martha J. Gdowski, Ph.D. and Neuroscience Graduate Program alumna Nguyen Mai, Ph.D. for being inducted into Alpha Omega Alpha, the national medical honor society! Election to Alpha Omega Alpha is an honor signifying a lasting commitment to professionalism, leadership, scholarship, research, and community service. A lifelong honor, membership in the society confers recognition for a physician's dedication to the profession and art of healing.
Third Annual Rochester Brain Bee sends Brighton High School Student to National Competition
Monday, February 4, 2019
 On Saturday, February 2, seven high school students competed for the title of Rochester's Brainiest Teenager. The students came from five schools in the Greater Rochester Area and competed in a grueling day with three rounds of 30 neuroscience questions in front of a panel of University of Rochester researchers. Ania Majewska, Ph.D., Chris Holt, Ph.D., Liz Romanski, Ph.D., Jude Mitchel, Ph.D., and Heather Natola, Ph.D., served as judges for the competition.
On Saturday, February 2, seven high school students competed for the title of Rochester's Brainiest Teenager. The students came from five schools in the Greater Rochester Area and competed in a grueling day with three rounds of 30 neuroscience questions in front of a panel of University of Rochester researchers. Ania Majewska, Ph.D., Chris Holt, Ph.D., Liz Romanski, Ph.D., Jude Mitchel, Ph.D., and Heather Natola, Ph.D., served as judges for the competition.
The quiz-style competition was structured so that each student had the opportunity to answer every question and the student with the highest cumulative score was declared the winner. The competition was won by Emily Han who correctly answered 69 out of 90 questions. Emily will receive a trip to the National Brain Bee in Hershey, Pa. in April, sponsored by the Rochester Society for Neuroscience and the Department of Neuroscience. The National Brain Bee will consist of two days of neuroscience and neurology questions on topics such as patient diagnosis, neuroimaging analysis, pathology, and general brain facts.
The Brain Bee was organized by the University of Rochester Brain Awareness Campaign Committee as a kick-off to 2019. The Rochester Brain Awareness Campaign was founded in 2013 and continues to offer neuroscience outreach for free to local schools. Since its founding, the team has visited 25 schools and reached 4,000 students with 200 volunteers. It has also had booth exhibits at Rochester Marches for Science, attended a St. John Fisher's science career day, and have had an official Rochester Girl Scouts badge every year since 2017. The organization is entirely student-run, and is supported by Ania Majewska, Ph.D., and the Rochester Society for Neuroscience.
Study suggests how high blood pressure might contribute to Alzheimer’s
Monday, January 28, 2019
The brain's system for removing waste is driven primarily by the pulsations of adjoining arteries, University of Rochester neuroscientists and mechanical engineers report in a new study. They also show that changes in the pulsations caused by high blood pressure slow the removal of waste, reducing its efficiency.
This might explain the association between high blood pressure and Alzheimer' disease, the researchers say. Alzheimer's, the most common cause of dementia among older adults, is characterized by abnormal clumps and tangled bundles of fibers in the brain.
The study, reported in Nature Communications, builds upon groundbreaking discoveries about the brain's waste removal system by Maiken Nedergaard, co-director of the University's Center for Translational Neuromedicine. Nedergaard and her colleagues were the first to describe how cerebrospinal fluid is pumped into brain tissue and flushes away waste. Subsequent research by her team has shown that this glymphatic waste removal system is more active while we sleep and can be damaged by stroke and trauma.
This latest research shows "in much greater depth and much greater precision than before" how the glymphatic system functions in the perivascular spaces that surround arteries in the outer brain membrane, says Douglas Kelley, an assistant professor of mechanical engineering and an expert in fluid dynamics. His lab is collaborating with Nedergaard's team as part of a $3.2 million National Institute on Aging grant.
For this study, Humberto Mestre, a PhD student in Nedergaard's lab, injected minute particles in the cerebrospinal fluid of mice, and then used two-photon microscopy to create videos showing the particles as they moved through the perivascular spaces.
In The News: UR study on brain waves may allow doctors to diagnose autism earlier
Friday, January 25, 2019
The following is an except of an article by Josh Navarro that originally appeared on WROC/RochesterFirst.com:
A new study to help understand brain waves in children with autism is underway right now at the University of Rochester Medical Center. Their aim is to foster earlier detection and foster better therapies in the future.
Children with autism respond differently when they hear a sound such as music or see an illustration. Honing in the difference in brain waves between autistic children and children who do not have autism, is part of a new study at Del Monte Institute for Neuroscience at URMC.
"If you can provide a biological marker that could be reproduced earlier in a child's development, then that therapy can start earlier, the better outlook for that particular child," said Dr. Evan Myers, Postdoctoral Fellow at the Cognitive Neurophysiology Lab in the University of Rochester Del Monte Institute for Neuroscience.
Researchers will place a electroencephalography cap and have kids observe different images on a computer screen. The findings will determine the next step through clinical trials with the goal of diagnosing a child with autism a lot sooner.
Common test of mental state understanding is biased
Thursday, January 24, 2019
The National Institute for Mental Health (NIMH) recommends a test, called the Reading the Mind in the Eyes Task (RMET). Here, participants view 36 black and white photographs, originally selected from magazine articles, of solely the eyes of Caucasian female and male actors. Participants then decide which of four adjectives—such as panicked, incredulous, despondent, or interested—best describes the mental state expressed in the eyes (the correct answer has been generated through consensus ratings).
But there's a problem. Using data from more than 40,000 people, a new study published this month in Psychological Medicine concludes that the test is deeply flawed.
"It's biased against the less educated, the less intelligent, and against ethnic and racial minorities," says lead author David Dodell-Feder, an assistant professor of psychology at the University of Rochester. "It relies too heavily on a person's vocabulary, intelligence, and culturally-biased stimuli. That's particularly problematic because it's endorsed by the national authority in our field and therefore the most widely-used assessment tool."
Dr. Kuan Hong Wang comes to the University of Rochester
Monday, January 21, 2019
We are pleased to welcome Dr. Wang to the University of Rochester Medical Center, the Department of Neuroscience and the Del Monte Institute for Neuroscience from the NIH.
Dr. Wang comes to us as the former chief of the Unit on Neural Circuits and Adaptive Behaviors at the National Institute of Mental Health. Dr. Wang received his B.A. in Biochemical Sciences from Harvard College and his Ph.D. from the University of California at San Francisco, where he studied the molecular regulators of sensory axon growth and branching during development with Marc Tessier-Lavigne. Dr. Wang obtained postdoctoral training with Susumu Tonegawa at the Massachusetts Institute of Technology, where he examined the ways in which cortical neurons respond to an animal's experience by directly visualizing the molecular activity of a given set of neurons over several days in the live animal. With this approach, he revealed a physiological function of neural activity regulated gene Arc in sharpening stimulus-specific responses in visual cortex.
