Cloning of RCP
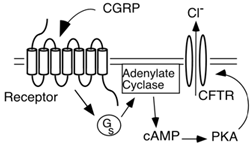
Figure 1A. CFTR is a Protein Kinase A (PKA)-
activated chloride channel, and when
co-expressed with a GCPR the CFTR produces a
ligand-induced chloride channel
We discovered RCP while conducting expression-cloning studies to identify the CGRP receptor. CGRP binding had been reported to result in elevated levels of intracellular cAMP, and the CGRP receptor was therefore thought to be a GCPR. However, standard expression cloning strategies did not yield a functional receptor, so we tried a method that did not rely on knowledge of receptor type for identification. We instead used a screen that used the cystic fibrosis transmembrane conductance regulator (CFTR) to detect elevated levels of cAMP.

Figure 1B. A single clone shown to facilitate
a CGRP-induced chloride current
The CFTR is a Protein Kinase A (PKA)-activated chloride channel, and when co-expressed with a GCPR the CFTR produces a ligand-induced chloride channel (Fig. 1A). We screened a cDNA library by in vitro transcribing the cDNA library and the CFTR, and co-injecting the library + CFTR cRNA into Xenopus laevis oocytes. Xenopus oocytes are large and can be patch-clamped, allowing detection of the chloride voltage induced by activation of the CFTR.
We isolated a single clone, which facilitated a CGRP-induced chloride current, but did not respond to other neuropeptides (Fig. 1B).

Figure 2. A short, hydrophilic (148 aa) protein
However, this protein did not appear to be a GCPR, as it was short (148 aa) and hydrophilic (Fig. 2). Furthermore, we found that this protein was intracellular, and associated with the cell membrane (Fig. 3). Thus, we postulated that it was part of a complex of proteins that together formed a functional receptor for CGRP, and named the protein the CGRP-receptor component protein (RCP). An alternate possibility was that RCP might not have been a receptor protein, but could have been a transcription factor that induced expression of the actual CGRP receptor. However, RCP worked in the oocyte-CFTR assay even with enucleated oocytes, or in the presence of α-amanitin (RNA polymerase II inhibitor), confirming that the effect we observed was not due to transcription.

Figure 3. Salt extraction of the hydrophilic protein in Figure 2.; illustrating that it is
intracellular, and associated with the cell membrane