Research Projects
Receptors for the calcitonin family of neuropeptides.
Work from a number of labs has elucidated the receptors for the calcitonin family of neuropeptides. Calcitonin (CT) can bind to the calcitonin receptor, but in the presence of RAMP1 the calcitonin receptor becomes a high-affinity amylin (AMY) receptor. When the calcitonin-like receptor (CLR) is expressed with RAMP1, a high-affinity CGRP receptor is formed. When CLR is expressed with RAMP2 or RAMP3 it binds adrenomedullin (AM) with high affinity. Additionally, intermedin (IM) can bind to CLR in the presence of either RAMP1,2 or 3. RCP is required for signaling at the CLR/RAMP receptor, and work in our lab is focused on determining the function and mechanism of RCP in CLR signaling and as a possible regulator of receptor function in vivo.
Cloning of RCP
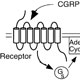 We discovered RCP while conducting expression-cloning studies to identify the CGRP receptor. CGRP binding had been reported to result in elevated levels of intracellular cAMP, and the CGRP receptor was therefore thought to be a GCPR. However, standard expression cloning strategies did not yield a functional receptor, so we tried a method that did not rely on knowledge of receptor type for identification. We instead used a screen that used the cystic fibrosis transmembrane conductance regulator (CFTR) to detect elevated levels of cAMP.
We discovered RCP while conducting expression-cloning studies to identify the CGRP receptor. CGRP binding had been reported to result in elevated levels of intracellular cAMP, and the CGRP receptor was therefore thought to be a GCPR. However, standard expression cloning strategies did not yield a functional receptor, so we tried a method that did not rely on knowledge of receptor type for identification. We instead used a screen that used the cystic fibrosis transmembrane conductance regulator (CFTR) to detect elevated levels of cAMP.
Learn more about Cloning of RCP
Function of RCP
 RCP co-immunoprecipitated with RAMP1 and CLR, suggesting that the functional CGRP receptor was a trimer of CLR/RAMP1/RCP proteins. RCP is highly conserved between species, yet does not contain obvious motifs that suggest how it might work to enable CGRP receptor function. Furthermore, RCP is expressed in all immortalized cell lines we have examined, even in those that do not express CGRP receptors and in most tissue, making gain-of-function experiments difficult.
RCP co-immunoprecipitated with RAMP1 and CLR, suggesting that the functional CGRP receptor was a trimer of CLR/RAMP1/RCP proteins. RCP is highly conserved between species, yet does not contain obvious motifs that suggest how it might work to enable CGRP receptor function. Furthermore, RCP is expressed in all immortalized cell lines we have examined, even in those that do not express CGRP receptors and in most tissue, making gain-of-function experiments difficult.
Learn more about Function of RCP
CGRP Biosynthesis
 Most neuropeptides are synthesized as large, biologically inactive precursors that must undergo a series of post-translational modifications to produce the smaller biologically active peptides. Modifications include endoproteolysis at pairs of basic amino acids, removal of the basic residues that constitute the cleavage site, carboxyl amidation and amino acetylation and cyclization. Endoproteolysis can exhibit tissue and developmental specificity, and is a key step in regulating peptide hormone secretion.
Most neuropeptides are synthesized as large, biologically inactive precursors that must undergo a series of post-translational modifications to produce the smaller biologically active peptides. Modifications include endoproteolysis at pairs of basic amino acids, removal of the basic residues that constitute the cleavage site, carboxyl amidation and amino acetylation and cyclization. Endoproteolysis can exhibit tissue and developmental specificity, and is a key step in regulating peptide hormone secretion.
Learn more about CGRP Biosynthesis
Carbon Nanotubes
 To facilitate high efficiency gene transfer into cells, arrays of carbon nanotubes are manufactured by template-based chemical vapor deposition (CVD), using commercially available anodized aluminum oxide membranes as template. These membranes contain pores, which determine the diameter and spacing of the carbon nanotubes. Carbon is deposited on all the surfaces of the membrane including the inner lumen of the pores, and after partial removal of the surface carbon and sacrificial membrane, the CNT tips are revealed. For our initial experiments we chose a Whatman AAO membrane with a pore diameter of 250 nm and a spacing of 200 nm between pores. The thickness of nanotube walls was controlled by CVD time, temperature and gas flow rate. After removing the excess carbon deposited on the membrane top surface with oxygen plasma, one side of the AAO membrane was partially etched with reactive ion etching (RIE) using boron trichloride to expose the tips of CNTs embedded in the membrane. The exposed CNT length was controlled by RIE etching time and RF power.
To facilitate high efficiency gene transfer into cells, arrays of carbon nanotubes are manufactured by template-based chemical vapor deposition (CVD), using commercially available anodized aluminum oxide membranes as template. These membranes contain pores, which determine the diameter and spacing of the carbon nanotubes. Carbon is deposited on all the surfaces of the membrane including the inner lumen of the pores, and after partial removal of the surface carbon and sacrificial membrane, the CNT tips are revealed. For our initial experiments we chose a Whatman AAO membrane with a pore diameter of 250 nm and a spacing of 200 nm between pores. The thickness of nanotube walls was controlled by CVD time, temperature and gas flow rate. After removing the excess carbon deposited on the membrane top surface with oxygen plasma, one side of the AAO membrane was partially etched with reactive ion etching (RIE) using boron trichloride to expose the tips of CNTs embedded in the membrane. The exposed CNT length was controlled by RIE etching time and RF power.