Major Lines of Investigation
Developing an Atlas of Gene Expression by Human Neural Stem and Progenitor Cells
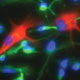 The biology of human brain cells differs substantially from that of laboratory animals. As a result, while the molecular data obtained from rodent models has been extraordinarily valuable in understanding basic cell and molecular biology of development, it has fallen short in identifying appropriate targets for therapeutic intervention; the predictive value of these models in modulating human brain function has proven marginal.
The biology of human brain cells differs substantially from that of laboratory animals. As a result, while the molecular data obtained from rodent models has been extraordinarily valuable in understanding basic cell and molecular biology of development, it has fallen short in identifying appropriate targets for therapeutic intervention; the predictive value of these models in modulating human brain function has proven marginal.
Learn more about Developing an Atlas of Gene Expression by Human Neural Stem and Progenitor Cells
Transcriptional and Pathway Regulation of Human Glial Tumor Stem Cells
 This project focuses on defining the gene e expression changes during anaplastic progression of isolated glioma tumor progenitor cells, derived and isolated during tumor progression from low grade gliomas through malignant gliomas and glioblastoma. By first isolating defined phenotypes of gliomas-initiating tumor stem cells from different staged tumors, then normalizing the gene expression patterns of these cells to that of normal glial progenitor cells derived from normal tissue, and then comparing those genes differentially expressed by glioma stem cells at each stage of tumor progression, to one another, this project seeks to define those genes dysregulated at all stages of glial tumorigenesis.
This project focuses on defining the gene e expression changes during anaplastic progression of isolated glioma tumor progenitor cells, derived and isolated during tumor progression from low grade gliomas through malignant gliomas and glioblastoma. By first isolating defined phenotypes of gliomas-initiating tumor stem cells from different staged tumors, then normalizing the gene expression patterns of these cells to that of normal glial progenitor cells derived from normal tissue, and then comparing those genes differentially expressed by glioma stem cells at each stage of tumor progression, to one another, this project seeks to define those genes dysregulated at all stages of glial tumorigenesis.
Learn more about Transcriptional and Pathway Regulation of Human Glial Tumor Stem Cells
Transcriptional Events in Remyelination by Human Oligodendrocyte Progenitors
 This project focuses on defining the gene expression changes associated with in vivo remyelination by human oligodendrocyte progenitor cells, using our novel human glial chimeric brain model. By way of background, little information is available as to the changes in gene expression or dominant pathway activation associated with the mobilization, oligodendrocyte fate commitment, or myelination by human oligodendrocyte progenitor cells during the process remyelination.
This project focuses on defining the gene expression changes associated with in vivo remyelination by human oligodendrocyte progenitor cells, using our novel human glial chimeric brain model. By way of background, little information is available as to the changes in gene expression or dominant pathway activation associated with the mobilization, oligodendrocyte fate commitment, or myelination by human oligodendrocyte progenitor cells during the process remyelination.
Learn more about Transcriptional Events in Remyelination by Human Oligodendrocyte Progenitors
Inducing Endogenous Progenitors as a Means of Treatment in Huntington’s Disease
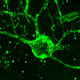 Besides my group’s efforts in glial progenitor cell biology and use, we have also a longstanding interest in adult neurogenesis and its potential induction for therapeutic purposes. In the course of my group’s longstanding work on adult neurogenesis in the songbird brain – a finding that I made as a graduate student many years ago and continue to investigate to the present day – we learned that two factors, the neurotrophic differentiation agent BDNF and the gliogenic suppressor noggin, are critical to permitting ongoing neuronal addition to adult brain tissue.
Besides my group’s efforts in glial progenitor cell biology and use, we have also a longstanding interest in adult neurogenesis and its potential induction for therapeutic purposes. In the course of my group’s longstanding work on adult neurogenesis in the songbird brain – a finding that I made as a graduate student many years ago and continue to investigate to the present day – we learned that two factors, the neurotrophic differentiation agent BDNF and the gliogenic suppressor noggin, are critical to permitting ongoing neuronal addition to adult brain tissue.
Learn more about Inducing Endogenous Progenitors as a Means of Treatment in Huntington’s Disease
Use of hiPSC and hESC-derived Neural Progenitors for Modeling Neuropsychiatric Disease
 Glial form and function are extraordinarily divergent with evolution, and human astrocytes are virtually unique in their pleomorphism and fiber complexity. In particular, human astrocytes are larger and more structurally complex than rodent glia, and coordinate the actions of vastly more synapses within their geographic domains. To assess the relative contributions of glial cells to the species-specific aspects of human cognition, we had previously engrafted neonatal mice with human glial progenitor cells (GPCs), to establish brains chimeric for human astrocytes.
Glial form and function are extraordinarily divergent with evolution, and human astrocytes are virtually unique in their pleomorphism and fiber complexity. In particular, human astrocytes are larger and more structurally complex than rodent glia, and coordinate the actions of vastly more synapses within their geographic domains. To assess the relative contributions of glial cells to the species-specific aspects of human cognition, we had previously engrafted neonatal mice with human glial progenitor cells (GPCs), to establish brains chimeric for human astrocytes.
Glial Progenitor-based Cell Therapy in Myelin Disease
 In the most clinically-advanced of the lab’s lines of investigation, we have established protocols for the identification, isolation and transplantation of human oligodendrocyte progenitor cells, which have succeeded in completely remyelinating the nervous systems of congenitally-hypomyelinated animals and rescuing normal neurological phenotype.
In the most clinically-advanced of the lab’s lines of investigation, we have established protocols for the identification, isolation and transplantation of human oligodendrocyte progenitor cells, which have succeeded in completely remyelinating the nervous systems of congenitally-hypomyelinated animals and rescuing normal neurological phenotype.
Learn more about Glial Progenitor-based Cell Therapy in Myelin Disease