McGrath Lab graduate student’s accidental exhale leads to improved DNA detector
Thursday, December 7, 2017
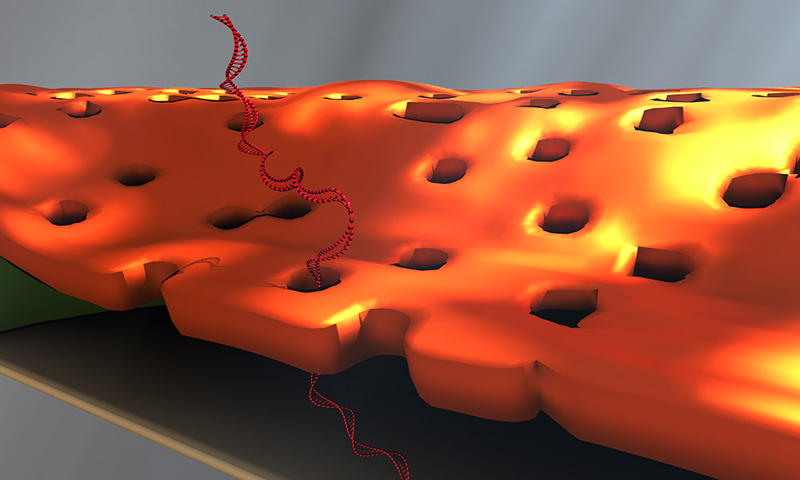
Doctoral student Greg Madejski's illustration of the layers comprising his new DNA detection device. (University of Rochester illustration / Greg Madejski)
Greg Madejski held his breath as he looked into the microscope, trying to weld two fingernail-sized chips together: a tiny chip containing a nanofilter on top of another chip with a DNA sensor.
It was frustrating work. The chips weren't making good contact with each other. Madejski gently poked at the chips, then peered over the top of the microscope.
And exhaled.
The sudden waft of warm air swept over the nanofilter, transferring it to the sensor—right on target. The "accident" led Madejski to an important insight: the water vapor in his breath had condensed on the device, causing the nanofilter to adhere ever so neatly to the sensor.
"It was like a really high-tech temporary tattoo that I created by accident; lick and stick!" says the PhD student in the lab of James McGrath, a professor of biomedical engineering at the University of Rochester.
And that's how water vapor became integral to the development and design of a novel device for detecting DNA biomarkers affiliated with disease. Created by McGrath's lab in collaboration with Professor Vincent Tabard-Cossa and graduate student Kyle Briggs at the University of Ottawa, the device is described in an article published online at Nano Letters. The article, and an image from Madejski's homemade animation of the device in operation, will be highlighted on the cover of the February 2018 print issue.
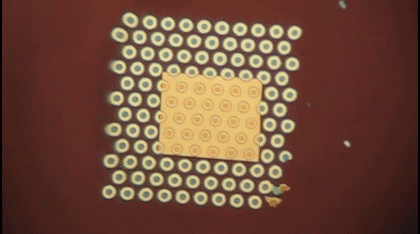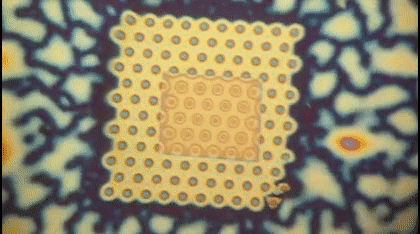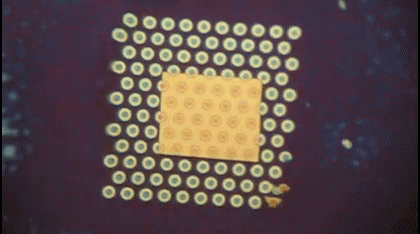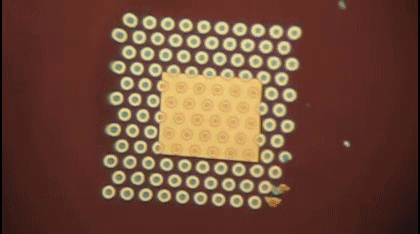
This animation shows, as graduate student Greg Madejski explains, the "thin films of water, seen as rainbow colors, swelling and shrinking the space between the prefilter and the nanopore as its exposed to additional water vapor.
'A remarkable structure'
The device is comprised of three ultrathin layers:
- a nanoporous silicon nitride membrane which serves as a prefilter.
- a biosensor membrane with a single nanopore.
- a spacer layer that separates these by only 200 nm.
The arrangement creates a nanocavity filled with less than a femtoliter of fluid—or about a million times smaller than the smallest raindrops.
During operation, the device uses an electric field to lure a strand of DNA to enter one of the pores of the prefilter and then pass through the nanocavity to reach the pore of the underlying sensor membrane. This triggers changes in the device's electrical current that can be detected and analyzed. The fact that DNA must elongate itself in a consistent way to pass through the two-membrane combination improves the precision and reproducibility of detection.
"This is a remarkable structure," says McGrath. "We've built an integrated system with a highly porous filter within molecular reach of a sensor. I think there are many sensors, particularly those that hunt for biomarkers in raw biological fluids, that would benefit from filtering away unwanted molecules immediately upstream of the detector."
The method of fabrication instantly wets the nanocavity, which is often difficult at the nanoscale. The device contains dozens of these nanocavities, which may eventually increase the amount of material that can be screened by enabling parallelized biomarker detection.
Solving problems that others need solved
Tabard-Cossa's lab uses solid-state nanopore devices to find new ways to manipulate and characterize single molecules. His lab was interested in finding new materials that could be used for biomarker detection. The prefilter in the new device addresses a problem with other silicon nanopore detectors: They are more likely to clog than alternative devices that use that biological pores for sensing. Biological membranes, on the other hand, are less stable than solid state nanopores, McGrath noted.
"We love to apply our membrane technologies to solve problems that others need solved. This is a very nice example.," McGrath says.
McGrath is co-founder of SiMPore, a University-based startup that develops highly portable, chip-based devices that incorporate silicon membranes for a variety of applications, from biological sensing to dialysis.
"I think we're going to realize the practical advantages of this technology in the near term," he says. A second generation of the new device, developed at SiMPore, incorporates the prefilter right on the chips during manufacturing at the wafer scale, "so there's nobody breathing on it anymore," he notes. "It's actually all built as one unit and should make future studies very easy. That's a credit to the ingenuity at SiMPore and quite a legacy for Greg."
URMC Awarded Nearly $6 Million to Study Deadly Bone Infections
Monday, November 6, 2017
Bone infection, while relatively rare, can be debilitating and potentially fatal. In recent years, researchers in the Center for Musculoskeletal Research at the University of Rochester Medical Center have made several discoveries that position them to advance new treatments and possible cures for bone infections. Now, a nearly $6 million, 5 year award from the National Institute of Arthritis and Musculoskeletal and Skin Disease at the National Institutes of Health, will allow the group to create a new multidisciplinary research program devoted to studying bone infections.
The CMSR has been among the top five NIH-funded orthopaedic research centers in the nation for over ten years, and Edward Schwarz, Ph.D., Burton Professor of Orthopaedics and director of the CMSR, has been the top NIH-funded orthopaedic researcher in the nation three years running. This new grant, awarded to Schwarz and throng of researchers from across the University of Rochester and beyond, brings the center's total forecasted earnings for 2017 to $28 million.
Of the millions of Americans who have joint replacement surgeries each year, less than five percent come away with an infection. But this minority of patients must endure a long and difficult road to recovery, if they recover at all. The vast majority of these infections are caused by a bacteria called Staphylococcus aureus, including the dreaded methicillin-resistant strain (MRSA), which causes sepsis and death in 13 percent of infected patients.
Patients who survive these infections face multiple surgeries to remove infected tissue, months of strong antibiotic treatments, and a high likelihood of re-infection. For a long time, researchers have been working to understand how this bacteria evades treatment and Schwarz believes he has figured out.
Together with Karen Bentley, director of the Electron Microscopy Core at URMC, Schwarz showed that the bacteria can crawl deep into tiny channels in bones, possibly taking shelter there and later emerging to re-establish an infection. Though S. aureus was originally thought to be incapable of movement, Bentley and Schwarz, in collaboration with James McGrath, Ph.D., professor of Biomedical Engineering at URMC, and his spin-off company, SiMPore Inc., showed that this bacteria can migrate through tiny pores in membranes in the lab.
This new grant will allow Schwarz and Hani A. Awad, Ph.D., professor of Biomedical Engineering and Orthopaedics in the CMSR, to investigate exactly how S. aureus gets into bone and develop new treatments that target those mechanisms. Microbiologists Steven Gill, Ph.D., and Paul Dunman, Ph.D., in the Department of Microbiology and Immunology, will help the team develop new antibiotics to inhibit bone infection, which will be 3D printed into spacers that replace infected joint implants. Delivering the antibiotic at the site of infection may save patients' limbs and lives.
Schwarz has also been working to understand what makes certain patients more susceptible to S. aureus infections than others, including why some patients recover relatively easily, while others die.
"Death following surgical site infection is not random," said Schwarz. "By studying patient immune responses to this bacteria, we might be able to predict who will be fine and who will need extra medical attention."
S. aureus can also become resistant to antibiotics, making it extremely deadly and difficult to eradicate. Better understanding patients' immune reactions to the bacteria may provide new approaches to defeating it.
In an international study of more than 300 patients with infected total joint replacements, Schwarz and his team including John Daiss, Ph.D., and Chao Xie, M.D., in the CMSR, found that patients fared well if their immune systems attacked a certain S. aureus protein, and poorly if they attacked another. Patients who produced antibodies attacking autolysin, a protein important for cell division, were protected. Those who produced antibodies against a family of iron sensing determinant (Isd) proteins, which help S. aureus sap nutrients from its host, were more likely to experience sepsis and even die.
It is unclear why antibodies that attack Isd proteins are bad for patients, and Schwarz is determined to use this new funding to figure it out. He will also analyze the full complement of antibodies produced by patients infected with several types of staph bacteria to see if there are more good- and bad-cop antibodies that could help inform new treatments.
The Clinical Research Core of this program will be run by Stephen L. Kates, M.D., at Virginia Commonwealth University.
Professor McGrath receives NIH funding for collaborative research project with the University of Ottawa
Tuesday, May 30, 2017
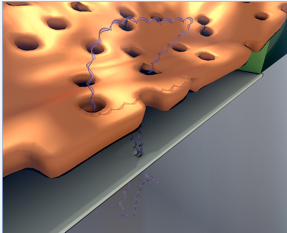
Nanoporous Nitride nanomembrane used as
a pre-filter for a DNA biosensor.
Professor Jim McGrath has received National Institutes of Health funding (R21) for his research project, “Solid-State Nanopores integrated with Nanoporous Membranes for enhanced Single-Molecule Counting of low-abundance Biomarkers,” in collaboration with the University of Ottawa.
This project aims to create robust biosensors by combining a University of Ottawa technology for the electrical detection of individual DNA molecules with University of Rochester’s nanomembrane technology. The porous nanomembranes serve as protective filters for the DNA sensors and prevent large molecules and debris from reaching the biosensor while still allowing the unobstructed passage of DNA. The technology will be used in a strategy in which designer DNA ‘barcodes’ serve as amplified surrogates for low abundance biomarkers present in biological fluids.
Professor McGrath receives NSF funding for research collaboration with RIT and SiMPore, Inc.
Friday, May 26, 2017

Figure: Concept of a compact extracorporeal dialysis
system enabled by silicon nanomembranes
A collaborative project including Rochester Institute of Technology, SiMPore, Inc., and BME Professor Jim McGrath titled, “Development of Ultrathin Nanomembranes for Home-based Hemodialysis,” has received National Science Foundation funding. This collaboration between University of Rochester, RIT, and SiMPore Inc. continues the development large area silicon nanomembranes for wearable hemodialysis. The high efficiency of the ultrathin nanomembranes enables new form factors for hemodialysis that can dramatically improve both quality of life and health outcomes for those with end stage renal failure.
BME PhD candidate Kilean Lucas takes second place at the Hajim School's Art of Science Competition
Saturday, May 6, 2017

Blood Cells by Scanning Electron Microscope

“You see some phenomenal things under a scanning electron microscope,” says Kilean Lucas.
Such as the image above, which the PhD student took of a single human red blood cell, captured among several white blood cells on a silicon nanomembrane developed in the lab of Lucas’ advisor, James McGrath, professor of biomedical engineering.
Lucas is studying how the ability of these nanomembranes to separate and filter out particles that differ by mere microns in size could lead to medical breakthroughs. For example, immature red blood cells separated from mature red blood cells could be harvested and seeded into bioreactors as a new way to replenish blood banks.
Or all the white blood cells could be filtered out from a patient’s blood sample. There are many forms of white blood cells, each responding in different ways to different threats. The proportion of each type of white blood cell in a given sample could be used to give a rough diagnosis of the body’s response to disease.
Lucas said it was not easy deciding which of several images from his research to submit to the Art of Science competition. “This one had a very good balance of being far enough out where we could see multiple types of cells, but not so close that we’re only looking at one or two,” Lucas says. “What I tried to do with the pseudo coloring is to emphasize that variety.”
He hopes this image will convey to people how “stunning it is, in and of itself, that we’re able to capture all of these cells out of blood, a very complex solution” – in ways that could change peoples’ lives.
Read more about this year's Art of Science Competition here.
Next stop for Falling Walls winner: Berlin
Friday, April 21, 2017

Biomedical engineering doctoral student Kilean Lucas delivers his presentation
at the annual Falling Walls competition. (University photo / Bob Marcotte)
Kilean Lucas admits to being uneasy about trying to summarize his research in three minutes, with only three slides.
You wouldn’t have known it yesterday, when Lucas, a PhD student in biomedical engineering, placed first at the University’s Falling Walls competition.
Lucas won $500 and an all-expenses paid trip to represent the University at the international Falling Walls competition in Berlin this fall.
Lucas described how the silicon nanomembranes developed in the lab of his advisor, James McGrath, could be used to filter out telltale exosomes (small, cell-derived vesicles) from the blood to provide early detection of cancer.
“There’s a lot of weight off my shoulders,” he said afterwards. “I was very nervous about coming to this and being able to communicate my project clearly in three minutes. That’s the hardest part of this.”
So how did he prepare?
With a big assist from his mentor and colleagues.
“I came up with the slides with help of professor McGrath,” Lucas said. Then I came up with a script that I tried out on my lab mates.”
Their feedback helped Lucas through four revisions of the script.
”Then I got together a group of people including my advisor and random people around the department and did a dry run. They gave me a lot of feedback,” Lucas said. In fact, he added, laughing, “ They tore my script apart. I completely reworked it yesterday.”
Sixteen competitors presented in front of a faculty panel that included jury chairman Richard Waugh, interim dean of faculty and associate vice president for research; Kim Arcoleo, associate dean for research at the School of Nursing; Dirk Bohmann, senior associate dean for basic research at the School of Medicine and Dentistry (SMD); Stephen Dewhurst, vice dean for research at SMD; Wendi Heinzelman, dean of the Hajim School of Engineering and Applied Sciences, and Joan Saab, chair of art and art history.
Runner up Kevin Mazurek, a post doctoral fellow in neurology, described a neurorehabilitative approach to help victims of stroke or other brain injury regain the ability to perform daily tasks.
Neurosurgery resident Jonathan Stone, the third place winner, described how patient-specific models of simulated tissue could help surgeons practice difficult procedures ahead of time.
The Falling Walls competition commemorates the fall of the Berlin Wall by giving young entrepreneurs and inventors from around the world the opportunity to express ideas about how to “break down the walls” hindering progress in dealing with challenges confronting science and society.
Lucas’ three-minute presentation:

Today I am going to talk to you about breaking the wall of cancer diagnostics.
Over the course of our lifetime, we have a 40 percent chance of developing cancer, and a 20 percent chance of dying of cancer. Late stage cancers present the highest risk of death as they are like a hydra.
Removing one tumor, or one head, does not guarantee that we kill the whole beast. In fact early diagnosis of cancer can save the lives of over 4 million people around the world each year.
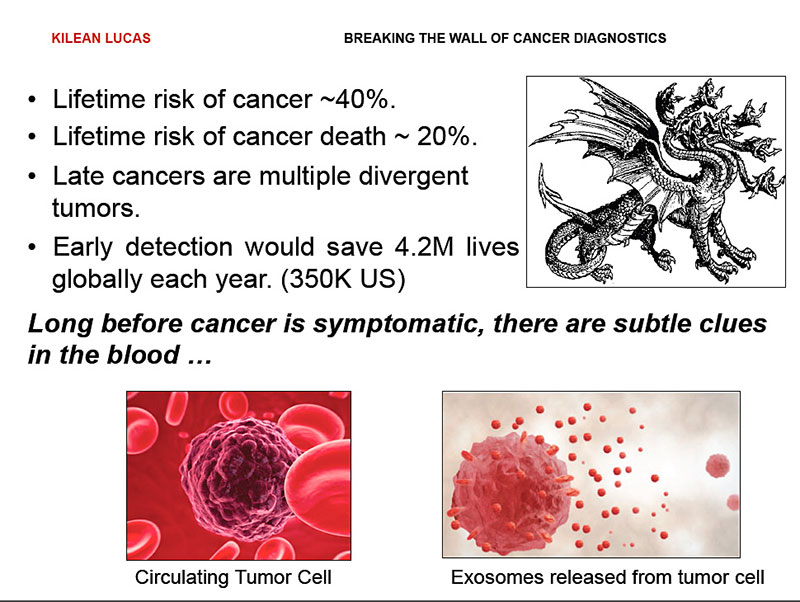
Conveniently, long before cancer presents outward symptoms, it releases subtle clues into the bloodstream in the form of circulating tumor cells and exosomes –50 nanometer vesicles that are basically breadcrumbs of information,
These exosomes contain micro RNA and proteins that are unique to the cells of origin, and provide us with a fingerprint of all the cells present in our body.
While they have all the information we need for diagnosis, searching for them is like searching for needle in haystack. Blood is a multicomponent system, and this makes it very difficult to easily search for these breadcrumbs.
However, what’s the best what to look for a needle in a haystack?
We need to filter out the hay.
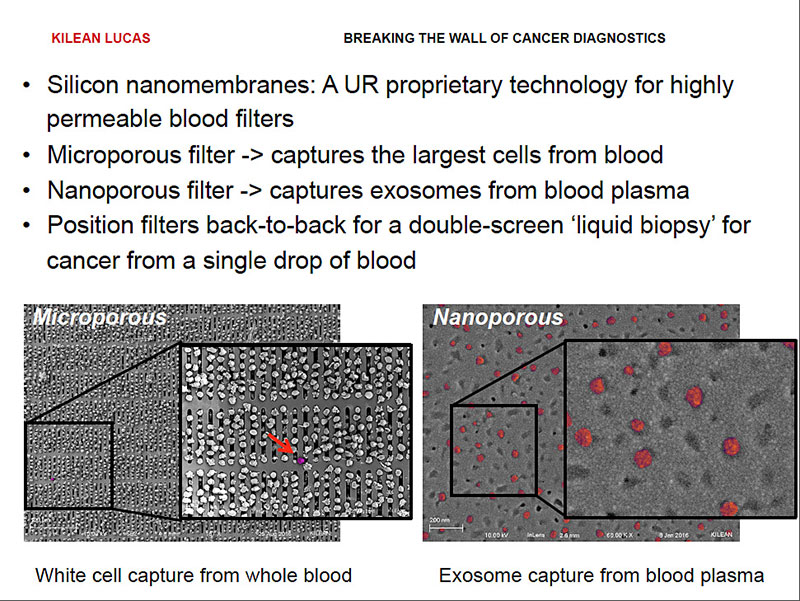
For blood, we have developed exactly the filters we need to do this.
Silicon nanomembranes are one-of-a kind-technology that has been developed right here at the University of Rochester. We have the ability to uniquely tune their pores for highly specific size cutoffs.
This allows us to take membranes with pores that are micrometers in size to completely remove all the cells from blood, including circulating tumor cells, leaving us with only the exosome containing plasma. We can then take that plasma and pass it over second membrane with nanometer sized pores, filtering out remaining proteins and capturing the exosomes.
By pairing these membranes back to back, we can incorporate them in a single device that we can put in front of a routine blood test, giving us an unobtrusive liquid biopsy for breaking the walls of early cancer diagnostics.