News
Cell Models Reveal a Shared Early Pathway to Macular Disorders
Wednesday, December 27, 2017
Ruchira Singh, PhD, of the University of Rochester is hopeful that different macular disorders could potentially be stopped by treatments aimed at some of the earliest cellular changes that these diseases have in common. This new understanding comes from her recent research showing there are similarities in the lead-up to different forms of macular degeneration that might lend themselves to shared treatment approaches.
It's good news, because shared therapies might be less costly to develop and use than separate therapies for each disease subtype, and could possibly be more effective at earlier stages.
Singh is using her 2015-18 Macular Degeneration Research (MDR) grant to study the role of different cells in the eye that are affected in age-related macular degeneration (AMD).
What makes her lab's work stand out, in particular, is that it replicates early aspects of macular degeneration in cell models created from living cells of adult humans. Whereas earlier forms of stem cell research relied on cells from embryonic tissue, this 21st century variation, called human induced pluripotent stem cell (hiPSC) technology, uses stem cells gathered from living adults, and then reprogrammed to differentiate into eye cells. iPSCs can self-renew and differentiate into nearly all cell types in the body.
Singh to study gene’s function in promoting retinal disease
Sunday, October 29, 2017
The retinal pigment epithelium (RPE) is a single layer of cells at the back of the eye that plays an indispensable role in supporting the light gathering photoreceptor cells that allow us to see. It is often thought of as a site where many blinding diseases begin, such as age related macular degeneration (AMD).
FEI Assistant Professor of Ophthalmology, Ruchira Singh, Ph.D., was recently awarded $1.95 million to study the role a gene called TIMP3 plays in regulating the extracellular matrix (ECM). The RPE's ECM provides structural and biochemical support to the RPE, which is necessary for normal function of RPE cells. In some retinal diseases, ECM abnormalities are linked to RPE dysfunction, suggesting that the ECM may be at the root.
Mutations to the TIMP3 gene, and its effect on the ECM, are responsible for Sorsby's Fundus Dystrophy (SFD). This disease closely mimics AMD. Singh, proposes to create a living tissue model of RPE-ECM using patient-derived human pluripotent stem cells (hiPSC) taken from subjects with SFD. To achieve this, she harvests skin cells from a person with SFD, re-programs them into stem cells and then differentiates them into unique cell types that model the RPE-ECM diseased cells.
Using this model, Singh will study the fundamental biology underlying SFD and the role that TIMP3 dysfunction plays in regulating a sequence of events that result in signs of SFD, such as the formation of drusen and the eventual growth of unwanted blood vessels in the retina. The knowledge gained in this study will help identify potential drug therapies for treating SFD. These may also prove useful in treating other retinal maculopathies such as AMD.
New Stem Cell Model Replicates Macular Degeneration
Tuesday, September 26, 2017
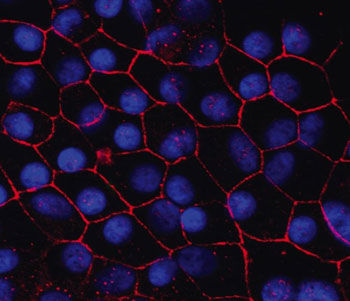
Human induced pluripotent stem cell-retinal pigment epithelium in a dish.
Macular degeneration is the leading cause of vision loss in older adults, but scientists have long struggled to study and replicate key elements of the disease in the lab. A study published in the Proceedings of the National Academy of Sciences is the first to demonstrate hallmarks of macular degeneration in a new human stem cell model developed by researchers at the University of Rochester Medical Center (URMC).
This new model could make whole new avenues of macular degeneration research possible and has helped the team hone in on some possible drug targets for the disease.
"So far, there has not been a patient-derived model of macular degeneration," said RPB Career Development Award recipient Ruchira Singh, PhD, assistant professor of Ophthalmology in the Flaum Eye Institute at URMC and lead author of the study. "It was not known if you can take cells from the human eye and make a cell model that displays the hallmarks of the disease."
New Model for Hard-to-Study Form of Blindness Paves Way for Future Research
Friday, September 15, 2017
Macular degeneration is the leading cause of vision loss in older adults, but scientists have long struggled to study and replicate key elements of the disease in the lab. A study published in the Proceedings of the National Academy of Sciences is the first to demonstrate hallmarks of macular degeneration in a new human stem cell model developed by researchers at the University of Rochester Medical Center.
This new model could make whole new avenues of macular degeneration research possible and has helped the team hone in on some possible drug targets for the disease.
"So far, there has not been a patient-derived model of macular degeneration," said Ruchira Singh, Ph.D., assistant professor of Ophthalmology in the Flaum Eye Institute at URMC and lead author of the study. "It was not known if you can take cells from the human eye and make a cell model that displays the hallmarks of the disease."
Though macular diseases can vary widely, age-related and similar inherited macular degenerative diseases are all characterized by buildup of debris in the retina, the light sensing tissue in the back of the eye that is crucial for vision. These deposits, called drusen, are specifically found beneath a layer of retinal pigment epithelium (RPE) cells, which are known to be key players in macular degeneration.
For their new model, Singh's team collected skin cells from patients with genetic forms of macular degeneration, re-programmed them to stem cells, and used the stem cells to create RPE cells. RPE cells derived from patients mimicked several characteristics of macular degeneration when aged in a dish, like producing the hallmark deposits.